Md Ali Akbar Khan
Department of Medicine, Dhaka Medical College Hospital, Dhaka, Bangladesh.
Md Titu Miah
Department of Medicine, Dhaka Medical College Hospital, Dhaka, Bangladesh.
Dr. Shah Mubdi-Un-Naafi
Department of Medicine, Dhaka Medical College Hospital, Dhaka, Bangladesh.
Keywords: Levofloxacin, Exfoliative dermatitis, Cat-2 Anti-TB drugs, Retreatment
DOI: 10.3329/bmrcb.v47i3.59247
Abstract
Background: Levofloxacin shows high bactericidal activity against mycobacterium tuberculosis with excellent safety profile. Rare adverse reactions include autoimmune haemolytic anaemia, hypoglycaemia, visual hallucinations, fixed drug eruptions, etc. Till date, only two cases of exfoliation associated with the Levofloxacin have been reported so far.
Objective: To review exfoliative dermatitis associated with the Levofloxacin and its prognosis in a retreatment case of mycobacterium tuberculosis.
The case: A 65 years old male was diagnosed as a case of Pulmonary TB, based on GeneXpert of Sputum examination. He was started on 1st line Anti-Tb drugs. No adverse drug reaction was reported at that time. Though he completed full 6 months course of anti-TB drugs, he was again tested positive for mycobacterium tuberculosis 2 months back and was started on Cat-2 Anti-TB drugs after being categorised as “RETREATMENT”. Just 20 days back before his admission to this hospital in May 2021, he developed cough. He also developed fever 7 days back and generalised itching with exfoliation of skin for 6 days. This cutaneous reaction occurred after starting Levofloxacin.
Conclusion: Immediately after detection, the responsible drug should be withheld, and treatment should be in a multi-disciplinary approach. Further studies are suggested for determination of potentials of cutaneous and other adverse reaction with the use of Levofloxacin along with 4-FDC in Rifampicin-sensitive retreatment category patients.
Keywords: Levofloxacin, Exfoliative dermatitis, Cat-2 Anti-TB drugs, Retreatment.
Introduction
Tuberculosis, a progressive granulomatous infectious disease, is accountable for 9 million new cases and 2 million deaths approximately around the world. Pulmonary TB as well as extrapulmonary TB are responsible for this huge burden. The South East Asia Region is the home to 39% of this global burden. Realising the socio-economic impact of this disease Govt of the People’s Republic of Bangladesh has been implementing DOTS strategy since 1993, through National Tuberculosis Programme.1 Though, there has been a rise in TB notification in Bangladesh since 2012, the NTP is working with a goal to end the TB epidemic, aiming to achieve a target of 10 new cases/ 100,000/year in 2035. (Projected 2015 baseline of 225 cases/100,000); which goes in line with SDG target 3.3.1–3
Cutaneous adverse drug reaction may complicate treatment with first line and 2nd line anti-TB agents; this belongs to type B adverse drug reaction (unpredictable, dose-independent). Examples include toxic epidermal necrolysis,fixed drug eruption, lichenoid drug eruption, drug-induced vasculitis; and drug hypersensitivity syndrome.4 Drug induced exfoliative dermatitis (ED) are a group of rare and severe drug hypersensitivity reactions involving skin and usually occurring from days to several weeks after drug exposure.5 World Health Organization has recommended fluoroquinolones as second-line agents for the treatment of multidrug-resistant TB and for cases with intolerance to first-line agents.6 TB Technical Committee of NTP has given directions regarding use of Levofloxacin for Retreatment Cases.7 Levofloxacin shows high bactericidal activity against mycobacterium tuberculosis, wide distribution, high penetration and excellent safety profile.8 Rare adverse reactions include autoimmune haemolytic anaemia, hypoglycaemia, visual hallucinations, fixed drug eruptions, etc. have been reported.9 Only 2 cases of exfoliation associated with the Levofloxacin has been reported till date.9,10 The report was aimed to review the exfolliative dermatitis associated with Levofloxacin and its prognosis in a retreatment case of mycobacterium tuberculosis.
The Case
A 65 years old male hailing from Narayanganj presented to covid unit of DMCH with the complaints of fever, cough and generalised exfoliation of skin for 6 days. He was reasonably well 10 months back. Then he was diagnosed as a case of Pulmonary TB on the basis of GeneXpert of sputum examination. He was started on 1st line Anti-TB drugs. No adverse drug reaction was reported at that time. Though he completed full 6 months course of Anti-TB drugs, he was again tested positive for mycobacterium tuberculosis 2 months back. Eventually he was started on Cat-2 Anti-TB drugs after being categorised as “RETREATMENT”. Just 20 days back before his admission to DMCH in May 2021, he developed cough. He also developed fever 7 days back and generalised itching with exfoliation of skin for 6 days. This cutaneous reaction occurred after starting Levofloxacin. In Cat-2 anti-TB regimen for pulmonary positive patient, TB Technical Committee, National TB Control Programme Bangladesh included Levofloxacin with 4FDC according to advice of WHO. On examination – investigation profile showed Hb - 12.5g/dL, WBC-17.17, Differential count – Neutrophil 50%, Eosinophil-34.7%, MCV -83.8 fL, MCH 27.4 pg, MCHC-32.7 g/dL . S.Ferritin was 517.7 ng/ml. CRP was <6 mg/L. D-dimer 1.30mg/L. RT PCR for COVID- 19 was negative. Sputum for GeneXpert revealed MTB, RIF resistance was not detected and MTB burden was low. S.Creatinine 0.9mg/dl. ALP 100 U/L. S. ALT -51U/L bilirubin 0.3 mg/dl. MT 02 mm.
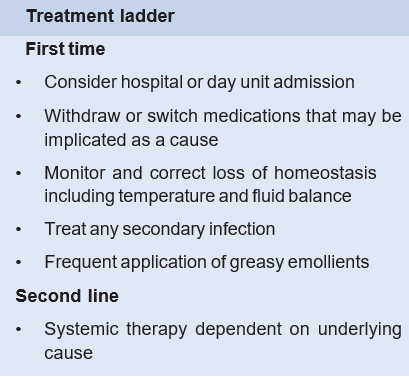
Biopsy was taken from the lesion for histopathological examination. On microscope, sections showed mild acanthosis and orthokeratosis. Some foci show thinning of dermal papillae. The upper dermis shows infiltration of eosinophils, lymphocytes and few plasma cells. No granuloma or malignancy was seen. Histopathological diagnosis was chronic non-specific dermatitis. The cutaneous condition was improved after discontinuing Cat-2 regimen.
Discussion
Exfoliative dermatitis is also known as dermatitis exfoliativa, pity•riasis rubra (Hebra), and erythroderma (Wilson-Brocq).11 Erythroderma is the term applied to any inflammatory skin disease that affects more than 90% of the body surface. Erythroderma is usually caused by exacerbation of a pre-existing dermatosis such as atopic dermatitis or psoriasis. Erythroderma can also be from severe drug reactions, idiopathic and less commonly CTCL.12 A wide range of drugs can cause erythroderma. Among the more commonly implicated are phenylbutazone, phenytoin, carbamazepine, cimetidine, gold salts and lithium.11
Levofloxacin has a well-established margin of safety and tolerability in community-acquired pneumonia, complicated urinary tract infections and in acute pyelonephritis.9 Several adverse effects with the use of levofloxacin e.g. Toxic Epidermal Necrolysis, DRESS Syndrome, Cutaneous leukocytoclastic Vasculitis, Acute Hyperpigmentation, Stevens- Johnson syndrome, Photosensitivity Mimicking Severe Cellulitis and Radiation recall dermatitis have been reported.13–23 A study showed there was no significant difference in the incidence of adverse effects between success group and failure groups in regard to treatment of MDR-TB.24 Even a study showed significantly lower adverse event rate in the levofloxacin-containing TB treatment regimens compared with the standard regimens.25 Till date, only two cases of exfoliative dermatitis with the use of levofloxacin have been reported.9,16 Gill et al showed a similar case like ours where there was generalised desquamation with the use of Levofloxacin.9 Levofloxacin was responsible for skin as they subsided with the withdrawal of drug. Same is the issue with our case; no such problems were seen when the patient was on cat-1 Anti-TB drugs;such exfoliation started with the introduction of levofloxacin in the retreatment protocol. Former is a representative of adult age group, but ours is representative of old age group.
Exfoliative dermatitis may retain the histologic features of the original disease process. Often, however, the histology is nonspecific, with hyperkeratosis, mild acanthosis, and focal parakeratosis.12 Histopathological report of our case also showed similar characteristics. Mechanism behind exfoliative dermatitis are apoptosis-inducing factors and lymphocyte-mediated cytotoxicity. Players behind keratinocyte death include - (1) Fas-FasL interaction, (2) Perforin/granzyme B pathway, (3) Granulysin and (4) Tumor necrosis factor a (TNF-a).5 (Figure 1)
Conclusion
Exfoliative dermatitis can be life threatening condition. As soon as it is detected, the responsible drug should be withheld and treatment should be in a multi- disciplinary approach. Further studies are now needed to determine the potentials of cutaneous and other adverse reaction with the use of levofloxacin along with 4-FDC in rifampicin-sensitive retreatment category patients because it can have a serious impact on health system as well as patient wellbeing.
References
- Khan MK, Islam N. An Overview on Epidemiology of Tuberculosis.
Available from: www.researchgate.net/publication/332875977 - Global SDG Indicator Platform.
Available from: sdg.tracking-progress.org/indicator/3-3-2-tuberculosis-incidence/ - National Tuberculosis Control Programme.
Available from: www.ntp.gov.bd/about-ntp/mission-and-vision/ - Lehloenya RJ, Dheda K. Cutaneous adverse drug reactions to anti-tuberculosis drugs: State of the art and into the future. Expert Review of Anti-Infective Therapy. 2012;10:475-486.
DOI: 10.1586/eri.12.13 - Yacoub MR, Berti A, Campochiaro C, et al. Drug induced exfoliative dermatitis: State of the art. Clinical and Molecular Allergy. 2016;14:9
DOI: 10.1186/s12948-016-0045-0 - Marra F, Marra CA, Moadebi S, et al. Levofloxacin treatment of active tuberculosis and the risk of adverse events. Chest. 2005;128:1406-1413
DOI: 10.1378/chest.128.3.1406 - National Tuberculosis Control Programme D.
DOI: www.ntp.gov.bd/2021/07/14/treatment-of-respiratory-tuberculosis-patients-4-fdc-and-levofloxacin-dosage/ - Van’t Boveneind-Vrubleuskaya N, Seuruk T, van Hateren K, et al. Pharmacokinetics of levofloxacin in multidrug- and extensively drug-resistant tuberculosis patients. Antimicrobial Agents and Chemotherapy. 2017;61. e00343-17.
DOI: 10.1128/AAC.00343-17 - Gill GK, Chhabra M, Chawla SPS. Levofloxacin-induced Desquamation: A Possible and Rare Case Report. Current Drug Safety. 2019;15:61-64.
DOI: 10.2174/1574886314666190708152223 - Ball P. Efficacy and Safety of Levofloxacin in the Context of Other Contemporary Fluoroquinolones: A Review. Current Therapeutic Research - Clinical and Experimental . 2003;64:646-661
DOI: 10.1016/j.curtheres.2003.11.003 - Arthur Rook. Rook’s Textbook of Dermatology. Vol 2. 9th ed. (Christopher E. M. Griffiths MD FFm, Jonathan Barker MD FFrcp, Tanya Bleiker FRCP, Robert Chalmers FRCP, Daniel Creamer MD F, eds.). Wiley Blackwell; 2016
DOI: - WILLIAM D. JAMES M, DIRK M. ELSTON M, JAMES R. TREAT M, MISHA A. ROSENBACH M, ISAAC M. NEUHAUS M. Andrews’ Diseases of the Skin.; 2020
DOI: - Uzun R, Yalcin AD, Celik B, Bulut T, Yalcin AN. Levofloxacin induced toxic epidermal necrolysis: Successful therapy with omalizumab (anti-IgE) and pulse prednisolone. American Journal of Case Reports. 2016;17:666-671.
DOI: 10.12659/AJCR.899823 - Yoon SY, Bae YJ, Cho YS, Moon HB, Kim TB. Toxic epidermal necrolysis induced by ofloxacin. Acta Dermato- Venereologica. 2010;90:550-551.
DOI: 10.2340/00015555-0912 - Varma SK, Sutradhar S, Misra AK. Levofloxacin and furazolidone induced toxic epidermal necrosis. Indian Journal of Pharmacology. 2013;45:625-626.
DOI: 10.4103/0253-7613.121380 - Digwood-Lettieri S, Reilly KJ, Haith LR, et al. Levofloxacin- induced toxic epidermal necrolysis in an elderly patient. Pharmacotherapy. 2002;22:789-793
DOI: 10.1592/phco.22.9.789.34074 - Charfi O, Lakhoua G, Sahnoun R, et al. DRESS Syndrome Following Levofloxacin Exposure with Positive Patch-test. Therapie. 2015;70:547-549.
DOI: 10.2515/therapie/2015046 - Blyth DM, Markelz E, Okulicz JF. Cutaneous leukocytoclastic vasculitis associated with levofloxacin therapy. Infectious Disease Reports. 2012;4:35-37.
DOI: 10.4081/idr.2012.e11 - Patil SS, Patil SM, Campbell R, Singh M, Plotkin M. Levofloxacin-Induced Acute Hyperpigmentation Changes in a Chronic Kidney Disease Patient. Case Reports in Medicine. 2020;2020.
DOI: 10.1155/2020/6186471 - Aversano MG, Schroeder J, Citterio A, et al. Levofloxacin induced Stevens Johnson syndrome/ toxic epidermal necrolysis overlap syndrome: case reports. Clinical and Translational Allergy. 2014;4(S3).
DOI: 10.1186/2045-7022-4-s3-p91 - Loupa C. Levofloxacin-Induced Photosensitivity Mimicking Severe Cellulitis in a Patient with Diabetic Foot Infection. MOJ Clinical & Medical Case Reports. 2017;7:311-313.
DOI: 10.15406/mojcr.2017.07.00221 - Wernicke AG, Swistel A, Parashar B, Myskowski P. Levofloxacin-induced radiation recall dermatitis: A case report and a review of the literature. Clinical Breast Cancer. 2010;10:404-406.
DOI: 10.3816/CBC.2010.n.054 - Gajjar K, Hirapara H, Jaiswal CS, Barvaliya M, Shah H, Tripathi CR. A Case Report of Antitubercular Drugs Induced Exanthematous Reaction Complicated by Acute Onset Levofloxacin Induced Toxic Epidermal Necrolysis (TEN). Current Drug Safety. 2018;13:41-43.
DOI: 10.2174/1574886311666160427104417 - Yew WW, Chan CK, Chau CH, et al. Outcomes of patients with multidrug-resistant pulmonary tuberculosis treated with ofloxacin/levofloxacin-containing regimens. Chest. 2000; 117:744-751.
DOI: 10.1378/chest.117.3.744 - Marra F, Marra CA, Moadebi S, et al. Levofloxacin treatment of active tuberculosis and the risk of adverse events. Chest. 2005;128:1406-1413.
DOI: 10.1378/chest.128.3.1406
Department of Medicine, Dhaka Medical College Hospital, Dhaka, Bangladesh.
smnaafi@gmail.com
 0000-0002-3272-1019
0000-0002-3272-1019
Submission
07 October 2021
Accepted
11 November 2021
Published
01 December 2021
Apply citation style format of Bangladesh Medical Research Council
Issue
Vol 47 No 3 (2021)
Section
Case Report
Financial Support
None
Conflict of Interest
None