Tania Islam Resma
Department of Virology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
Munira Jahan
Department of Virology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
Raad Rahmat
Department of Virology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
Shahina Tabassum
Department of Virology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
Keywords: Micro RNA 122, Real time PCR, Alphafeto protein, HBV DNA.
DOI: 10.3329/bmrcb.v47i3.59245
Abstract
Background: MicroRNA-122 (miR-122) is the most abundant liver-specific microRNA in humans which is released into the blood circulation in significant amounts from injured liver tissues. This circulating miRNA-122 can differentiate between chronic HBV carriers with high and low risks of disease progression.
Objective: To estimate the plasma levels of microRNA-122 in different groups of chronic hepatitis B (CHB) patients in Bangladesh.
Methods: The study consisted of 70 participants which included 20 CHB patients with hepatocellular carcinoma (HCC), 20 CHB patients with cirrhosis, 20 CHB patients without HCC and cirrhosis, along with 10 healthy controls (HC). Total RNA was extracted from plasma samples followed by cDNA synthesis and finally level of miR-122 was analysed using real time PCR technique.
Results: Level of miR-122 in plasma samples was elevated considerably in all patient groups under study compared to the healthy controls. It was 63.28±18.52 (mean ±SEM), 32.8±7.37 (mean ±SEM), and 125.84±24.8 (mean ±SEM) folds higher in CHB group with HCC, CHB group with cirrhosis and CHB group without HCC and cirrhosis respectively than that in the healthy control group. Furthermore, the plasma miR-122 level correlated positively with HBV DNA viral load in all groups of CHB patients but had no significant correlation with serum ALT and AFP levels. The receiver-operator characteristic (ROC) curves of plasma levels of miR-122 were generated to determine the specificity and sensitivity of this micro RNA to distinguish the patient groups from healthy controls. The plasma miR-122 showed sensitivity and specificity of 95% and 90%, 85% and 90%, 100% and 90% respectively when differentiating CHB group with HCC, CHB group with cirrhosis and CHB group without HCC and cirrhosis from healthy controls.
Conclusion: It may be concluded that, plasma level of miR-122 was increased in all the patient groups with CHB infection and correlated positively with HBV DNA viral load.
Keywords: Micro RNA 122, Real time PCR, Alphafeto protein, HBV DNA.
Introduction
MicroRNA-122 (miR-122) is an endogenous, small (22 nucleotides long), non-coding RNA molecule with a high level of expression in normal hepatocytes. It plays a pivotal role in myriads of liver functions including the growth, development and neoplastic transformation of the liver, along with lipid metabolism.1 In hepatitis, miR-122 is released into the circulation in significant amounts.2-4 Prior to release, they are packaged into apoptotic bodies, exosomes and other secretory particles. Furthermore, they are shown to have resistance against endogenous RNase activity, extremes of pH, high temperature and multiple freeze- thaw cycles, suggesting their credibility to serve as a potential marker in cancer detection.5,6 An estimated 257 million people are infected with hepatitis B virus (HBV), as they tested positive for the hepatitis B surface antigen, resulting in 887,000 deaths, globally in 2015, primarily from complications like cirrhosis and hepatic carcinoma.7 In terms of prevalence, Bangladesh categorises into the intermediate region for HBV infection. Currently, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are used as markers for liver injury, however, given their instability in extreme conditions and lack of tissue specificity, more sensitive and stable biomarkers with high degrees of specificity are required.8 MicroRNA appears to be a potential biomarker than ALT or AST in diagnosing virus induced liver damages with sufficient sensitivity and specificity. Both apoptosis and necrosis lead to hepatocyte cell death in hepatitis. During apoptosis, the hepatocytes synthesise less and thus, release fewer AST and ALT as well, but cellular microRNAs are released directly into the circulating system following both apoptosis and necrosis.9 This possibly demonstrates serum microRNA’s higher sensitivity in diagnosing liver damage than ALT or AST.
At present, only advanced stages of HCC can be diagnosed using biopsy, imaging (abdominal USG, MRI, and CT), along with a– fetoprotein (AFP). AFP is the most widely used and broadly known biomarker for HCC. However, in 35% of the cases, early stages of HCC (tumors less than 3cm) are missed during AFP analysis.10 Therefore, more sensitive and specific biomarkers are essential for early diagnosis and prognosis of HCC. Since MicroRNA-122 is the most abundant liver specific microRNA with stable expression in plasma can serve as a novel, non- invasive biomarker for the early detection and prognosis of HCC. The median levels of plasma miR- 122 are significantly higher in patients with CHB infection, cirrhosis, and HCC than those in healthy control, with the levels being the highest in patients with CHB infection. This indicates that elevated expression of miR-122 may be associated with liver damage caused by liver cancer and particularly, CHB infection.11,12 Plasma microRNA-122 also correlates with biochemical parameters of hepatocellular damage, liver function, and synthetic capacity.13
Materials and Methods
This cross sectional study was conducted during July 2017 to June 2018 among three different groups of chronic HBV infected (CHB) patients to see the level of miR-122 in different stages of hepatitis. Three different groups consisting of CHB patients with HCC, CHB patients with cirrhosis of liver and CHB patients without HCC and cirrhosis. Each group comprising 20 patients. Ten healthy controls were also enrolled in this study. CHB patients with HCC and cirrhosis were recruited from inpatient department of hepatology, Bangabandhu Sheikh Mujib Medical University (BSMMU). CHB patients without HCC and cirrhosis were recruited from outpatient department of Hepatology, BSMMU. MD Residents and staffs of department of virology were the healthy volunteers. All Patients with histories and clinical features of alcoholism, obesity, drug induced hepatotoxicity, NAFLD, steatohepatitis, diabetes mellitus as well as those treated with antiviral drugs were excluded from the study.
Blood samples were collected using aseptic venipuncture technique. The samples were labeled and case numbers were recorded on the clinical data sheet immediately. Informed, written consent were taken from each patient. Plasma were separated into two aliquots. One was kept in -20p c for HBV-DNA and miR-122 estimation. Another aliquot was kept for ALT and AFP analysis. HBV-DNA and miR-122 estimation were carried out in the Department of Virology, BSMMU. ALT and AFP test were done in Department of Biochemistry/Microbiology, BSMMU.
RNA extraction, reverse transcription and quantification by real-time PCR: Total RNA was extracted from 300 µl of plasma with the mirVana PARIS RNA and native protein quantification kit (Invitrogen Ambion) according to the manufacturer’s protocol. The extracted RNA was eluted in 100 µl of preheated elution solution and was assessed by measuring the absorbance at 260 and 280 nm with Nanodrop-2000 UV-Vis spectrophotometer. The total RNA samples were immediately stored at -20°c for cDNA synthesis.
500 ng/µl of total RNA including microRNAs from each sample was reverse transcribed with a miRNA specific and U6snRNA specific stem loop RT primer mix (Cohesion Biosciences, London, UK). The reactions were run in a thermocycler under the following conditions: 25°C for 30 minutes, 42°C for 30 minutes, 85°C for 5 minutes and ultimately held at 4°C. The resultant cDNA were stored at -20°c prior to quantification by real-time PCR (Cohesion Biosciences, London, UK). According to manufacturer’s instructions the PCR reaction was carried out in Step One real-time PCR system (Applied Biosystems) under the following conditions: (hold at 95°c for 3 minutes, 40 cycles of 95°c for 12 seconds and 62°c for 40 seconds). U6snRNA was taken as a reference for normalization of the expression levels of the target miRNA. The miRNA relative expression levels were calculated by the 2-△△Ct method and data were analyzed using equation where △△Ct= (Ct target– Ct U6snRNA) target group – (Ct target – CtU6snRNA) control group.
Statistical analysis
The mean ± SEM of fold changes were determined from the samples of all groups. Comparisons between groups were done by the Mann-Whitney U test. Non parametric Kruskal Wallis H test was performed for multiple comparisons. The correlation of plasma miR- 122 levels with the viral load, ALT and AFP levels were analysed by Spearman correlation test. Statistical analysis was made using SPSS 21 software. p value of <0.05 was considered statistically significant.
Results
Among the 70 participants, 63 were male and seven (7) were female. The mean age was 35 years. Most of the patients were from rural area. Maximum of the patients were businessmen who belonged to middle class of the society (table I).
|
Characteristics |
Category |
Frequency |
|---|---|---|
|
Age |
20-40 years |
44 (63%) |
|
|
41-60 years |
17 (24%) |
|
|
61-80 years |
09 (13%) |
|
Sex |
Male |
63 (90%) |
|
|
Female |
07 (10%) |
|
Demographic area |
Rural |
39 (56%) |
|
|
Urban |
31 (44%) |
|
Occupation |
Farmer |
06 (9%) |
|
|
Service holder |
13 (19%) |
|
|
Businessman |
40 (57%) |
|
|
Housewife |
02 (3%) |
|
|
Student |
09 (12%) |
|
Socioeconomic status |
Lower class (<5000 tk.) |
11 (16%) |
|
(monthly income) |
Middle class (5001-30000 tk.) |
56 (80%) |
|
|
Upper class (>30000 tk.) |
03 (04%) |
|
Religion |
Muslim |
64 (91%) |
|
|
Hindu |
06 (09%) |
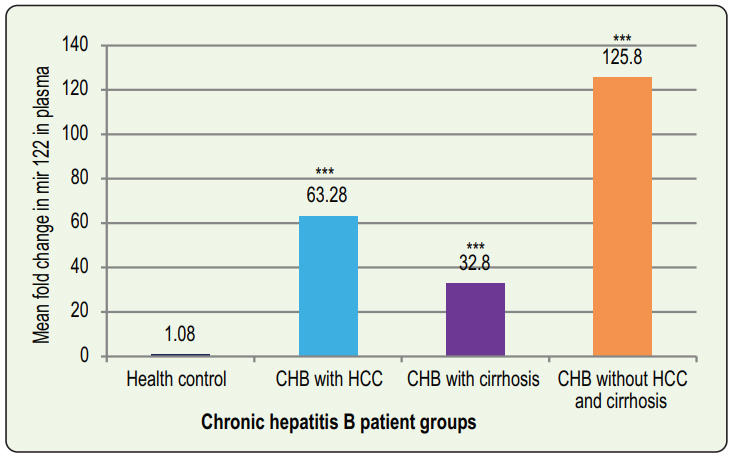
The miR-122 level in CHB without HCC and cirrhosis group was 125.8±24.8 (mean ±SEM) fold higher than the HC group. The mean fold change of miR-122 in the HC group was 1.08±0.14 (mean ±SEM). The difference of miR-122 fold change were statistically significant among the four groups: p≤0.01 (Kruskal Wallis H test) and between groups: p≤0.01, except CHB group with HCC vs CHB group with cirrhosis: p = 0.38 (Mann-Whitney U test). The mean miR-122 level was found to be 63.28 ± 18.52 (mean ± SEM) and 32.8 ± 7.37 (mean ± SEM) fold higher than healthy control in CHB patients with HCC and Cirrhosis groups respectively (Figure 1).
In patients of CHB with HCC group, the mean viral load was 5.04 ±0.29 (mean ±SEM) log10 copies/ml, ALT level was 56.3±5.9 (mean ±SEM) IU/Land AFP level was 34867.2±18383.5 (mean ±SEM) ng/ml. The correlation analyses were done using Spearman correlation analysis. A positive correlation was observed between plasma miR-122 level and HBV- DNA level (table II).
|
Groups |
Variables |
r value |
pvalue |
|
|---|---|---|---|---|
|
CHB with HCC (n=20) |
miR-122 |
HBV DNA log10 copies/ml |
0.881 |
0.000* |
|
|
ALT (IU/L) |
-0.202 |
0.393ns |
|
|
|
AFP (ng/ml) |
-0.028 |
0.907ns |
|
|
CHB with cirrhosis (n=20) |
miR-122 |
HBV DNA log10 copies/ml |
0.893 |
0.000* |
|
|
ALT (IU/L) |
0.022 |
0.927ns |
|
|
|
AFP (ng/ml) |
0.171 |
0.472ns |
|
|
CHB without HCC and cirrhosis (n=20) |
miR-122 |
HBV DNA log10 copies/ml |
0.690 |
0.001* |
|
|
ALT (IU/L) |
0.010 |
.967ns |
|
|
|
AFP (ng/ml) |
0.305 |
.190ns |
|
Note: n = numbers of patients in a group. Correlation was established by Spearman’s correlation coefficient
test. r = correlation coefficient
* = statistically significant ns = non-significant.
A negative correlation was observed between plasma miR-122 level and ALT and between plasma miR-122 and AFP. In CHB with cirrhosis group, the mean viral load was 4.86±0.26 (mean ±SEM) log10 copies/ml, ALT level was 147.2±39.8 (mean ±SEM) IU/L and AFP level was 6570.3 ±5140 (mean ±SEM) ng/ml. A positive correlation was observed between plasma miR-122 level and HBV-DNA. A weak positive correlation was found between plasma miR-122 level and ALT and between plasma miR-122 and AFP (table II).
In CHB without HCC and cirrhosis group, the mean viral load was 6.84±0.27 (mean ±SEM) log10 copies/ ml, ALT level was 60.55±9.04 (mean ±SEM) IU/L and AFP level was 3.33±0.74 (mean ±SEM) ng/ml. A positive correlation was observed between plasma miR- 122 and HBV-DNA level. A weak positive correlation was found between plasma miR-122 level and ALT and also between plasma miR-122 and AFP (table II).
|
Groups |
Area under curve (AUC) |
Standard Error |
P value |
Sensitivity |
Specificity |
Cut-off value |
|---|---|---|---|---|---|---|
|
CHB with HCC |
0.995 |
0.008 |
0.000 |
95% |
90% |
1.8 |
|
CHB with cirrhosis |
0.965 |
0.029 |
0.000 |
85% |
90% |
1.82 |
|
CHB without HCC |
1.000 |
0.000 |
0.000 |
100% |
90% |
1.8 |
|
and cirrhosis |
|
|
|
|
|
|
Receiver Operating Characteristic (ROC) curve analysis was performed to evaluate the sensitivity and specificity of plasma miR-122 in distinguishing the different groups of CHB patients from healthy controls. Comparing healthy controls with CHB group with HCC, AUC of plasma miR-122 was found to be 0.995 (95% CI: 0.979-1.000), with CHB group with cirrhosis, 0.965 (95% CI: 0.908-1.000) and with CHB group without HCC and cirrhosis was 1.000 (95% CI: 1.000-1.000). At the cut-off value 1.8, the sensitivity and specificity of the tests for CHB group with HCC, with cirrhosis and without HCC and cirrhosis were 95% and 90%, 85% and 90%, 100% and 90% respectively (table III).
Discussion
Chronic HBV infection can alter miR-122 expression in liver. As this miRNA is released in huge amount into the circulation after liver injury, the plasma level of it may be a reflection of necro-inflammatory changes taking place in liver tissues. A significant difference in levels of miR-122 in plasma of different groups of CHB patients indicates that it can be used as a biomarker of liver disease progression.14-16 Therefore, the objectives of the present study were to determine the plasma levels of miR-122 among CHB patients and to correlate this with other surrogate markers of liver diseases like viral load, ALT and AFP levels of them. In the present study, levels of miR-122 in plasma were significantly higher in CHB patient groups than in HC group (figure 1). Chronic HBV carrier group showed highest fold change of miR-122 among the patient groups. Chronic liver inflammation, necrosis and release of miR-122 from hepatocyte causes highest fold change of miR-122 in this group of patients. The reason of lower plasma miR-122 concentration among CHB groups with HCC and cirrhosis may be due to the lessening of functioning liver tissues in advanced liver diseases. Waidmann et al, performed a study on cirrhotic patients and observed lower levels of miR- 122 in patients with more advanced diseases, indicating that reduced serum miR-122 is most likely the result of reduced release from hepatocytes and that miR-122 levels are associated with hepatic functional capacity.15 In their study, Xing et al showed that the expression levels of miR-122 in serum of patients with HCC and CHB were significantly higher than those in the cirrhosis and asymptomatic carrier groups.11
The present study observed a positive correlation between plasma miR-122 level and HBV DNA in all the patient groups, this positive correlation links active viral replication and associated necroinflammatory changes of liver. MiR-122 was negatively correlated with both ALT and AFP in CHB group with HCC and weakly positive in both CHB group with cirrhosis and Chronic HBV carrier group (table I). Xing et al, showed a positive correlation of plasma miR-122 with HBV DNA but no significant correlation between the levels of miR-122 and ALT or AFP among CHB patients. ROC curve analyses performed to measure the diagnostic accuracy of plasma miR-122, yielded 95% sensitivity and 90% specificity when differentiating CHB group with HCC from HC, 85% sensitivity and 90% specificity when differentiating CHB group with cirrhosis from HC and 100% sensitivity and 90% specificity when differentiating Chronic HBV carrier group from healthy controls (table II). This result indicated that miR-122 can be used as a biomarker with good sensitivity and specificity. At present, AFP level is considered as a useful tumor marker for HCC diagnosis.16,17
Conclusion
In the present study, plasma level of miR-122 was found to be increased in all the patient groups with CHB infection but the highest fold change was observed in CHB patients without HCC and cirrhosis. Furthermore, plasma miR-122 levels correlated positively with HBV DNA viral load in all the patient groups. In the study, this miRNA achieved good sensitivity and specificity in distinguishing patient groups from healthy controls. Therefore, it may be concluded that, plasma miR-122 is sensitive and specific enough to be used as an easily accessible new potential marker for differentiation of chronic hepatitis B patients from healthy individuals.
References
- Girard M, Jacquemin E, Munnich A, Lyonnet S and Henrion- Caude A. miR-122, a paradigm for the role of microRNAs in the liver. Journal of Hepatology. 2008; 48: 648-656.
DOI: 10.1016/j.jhep.2008.01.019 - Qi P, Cheng SQ, Wang H, Li H, Chen YF and Gao CF. Serum microRNAs as biomarkers for hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infection. PloS One. 2011; 6: e28486: 1-8.
DOI: 10.1371/journal.pone.0028486 - Arroyo JD, Chevillet JR, Kro EM, Ruf IK, Pritchard CC, Gibson, DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proceedings of the National Academy of Sciences. 2011; 108: 5003-5008.
DOI: 10.1073/pnas.1019055108 - Novellino L, Rossi RL, Bonino F, Cavallone D, Abrignani S, Pagani M et al. Circulating hepatitis B surface antigen particles carry hepatocellular microRNAs. PloS One. 2012; 7: e31952: 1-10.
DOI: 10.1371/journal.pone.0031952 - Jiang L, Cheng Q, Zhang BH and Zhang MZ. Circulating microRNAs as biomarkers in hepatocellular carcinoma screening: a validation set from China. Medicine. 2015; 94: 603.
DOI: 10.1097/md.0000000000000603 - Rechavi O, Erlich Y, Amram H, Flomenblit L, Karginov FV, Goldstein, et al. Cell contact-dependent acquisition of cellular and viral nonautonomously encoded small RNAs. Genes & Development. 2009; 23: 1971-1979.
DOI: 10.1101/gad.1789609 - World Health Organization Hepatitis B. Fact sheet [updated July 2017]. Available from: https://www.who.int/news- room/fact-sheets/detail/hepatitis-b
DOI: - Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J, et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug induced, and inflammatory liver diseases. Hepatology. 2012; 56: 1946-1957.
DOI: 10.1002/hep.25873 - Zhang H, Li QY, Guo Z, Guan Y, Du J, Lu YY, et al. Serum levels of microRNAs can specifically predict liver injury of chronic hepatitis B. World Journal of Gastroenterology. 2012; 18: 5188-5196.
DOI: 10.3748/wjg.v18.i37.5188 - Saffroy R, Pham P, Reffas M, Takka M, Lemoine A and Debuire B. New perspectives and strategy research biomarkers for hepatocellular carcinoma. Clinical Chemical Laboratory Medicine. 2007; 45: 1169-1179.
DOI: 10.4254/wjh.v7.i2.139 - Xing TJ, Jiang DF, Huang JX and Xu ZL. Expression and clinical significance of miR-122 and miR-29 in hepatitis B virus-related liver. Genetics and Molecular Research. 2014; 13: 7912-7918.
DOI: 10.4238/2014.september.29.4 - Xu J, Wu C, Che X, Wang L, Yu D, Zhang T, et al. Circulating MicroRNAs, miR 21, miR 122, and miR 223, in patients with hepatocellular carcinoma or chronic hepatitis. Molecular Carcinogenesis. 2011; 50: 136-142.
DOI: 10.1002/mc.20712 - Jin BX, Zhang YH, Jin WJ, Sun XY, Qiao GF, Wei YY, et al. MicroRNA panels as disease biomarkers distinguishing hepatitis B virus infection caused hepatitis and liver cirrhosis. Scientific Reports. 2015; 5:15026-15038.
DOI: 10.1038/srep15026 - Waidmann O, Bihrer V, Pleli T, Farnik H, Berger A, Zeuzem S et al. Serum microRNA 122 levels in different groups of patients with chronic hepatitis B virus infection. Journal of Viral Hepatitis. 2012; 19: 58-65.
DOI: 10.1111/j.1365-2893.2011.01536.x - Zhang Y, Jia Y, Zheng R, Guo Y, Wang Y, Guo H, et al. Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clinical Chemistry. 2010; 56: 1830-1838.
DOI: 10.1373/clinchem.2010.147850. - Zhang BH, Yang BH and Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. Journal of Cancer Research and Clinical Oncology. 2004; 130: 417-422.
DOI: 10.1007/s00432-004-0552-0 - Leandro G, Zizzari S, Fabris C, Basso D, Elba S, Del Favero G, et al. Do CA 19-9 and TPA play a minor role as compared to AFP in diagnosing primary hepatocellular carcinoma? Oncology. 1989; 46: 381-385
DOI: 10.1159/000226756
Department of Virology, Bangabandhu Sheikh Mujib Medical University, Shahbagh, Dhaka, Bangladesh.
munirajahan@bsmmu.edu.bd
 0000-0003-3935-9013
0000-0003-3935-9013
Submission
27 December 2020
Accepted
17 October 2022
Published
01 December 2021
Apply citation style format of Bangladesh Medical Research Council
Issue
Vol 47 No 3 (2021)
Section
Research Articles
Ethical Clearance
National Research Ethics Committee of BMRC, Dhaka, Bangladesh.
Financial Support
Bangladesh Medical Research Council (BMRC), Dhaka, Bangladesh.
Conflict of Interest
There is no conflict of Interest.