Choman Abdullah Mohana
COVID-19 unit, Mymenshing Medical College Hospital, Mymenshing, Bangladesh.
M A Hasanat
Department of Endocrinology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh.
Emran Ur Rashid
Department of Endocrinology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh.
Iffat Ara Jahan
Department of Endocrinology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh.
Md Shahed Morshed
Kurmitola General Hospital, Dhaka, Bangladesh.
Hurjahan Banu
Department of Endocrinology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh.
Sharmin Jahan1
COVID-19 unit, Mymenshing Medical College Hospital, Mymenshing, Bangladesh.
Keywords: Leptin, Adiponectin, Leptin/adiponectin ratio, Polycystic ovary syndrome
DOI: 10.3329/bmrcb.v47i3.59241
Abstract
Background: Polycystic ovary syndrome (PCOS) is a common endocrine problem and increasing worldwide. Dysregulated adipocytokine production is thought to be linked with pathogenesis of PCOS.
Objective: The aim of this study was to see the relation of leptin, adiponectin and their ratio with PCOS and its cardiovascular risks.
Methods: This case-control study included 20 PCOS patients diagnosed by revised Rotterdam criteria, 2003 and equal number of age matched control. Relevant clinical information was collected in datasheet. Blood was taken to measure glucose at fasting and after oral glucose tolerance test (OGTT), total testosterone (TT), sex hormone binding globulin (SHBG), luteinizing hormone (LH), follicle stimulating hormone (FSH), insulin, fasting lipid profile, leptin and adiponectin from each participant. Glucose was measured by glucose oxidase, all hormones by chemiluminescent immunoassay, lipids by glycerol phosphate dehydrogenase-peroxidase and leptin as well as adiponectin by enzyme linked immunosorbent assay.
Results: Leptin (ng/ml) [30.0 (24.15, 68.13) vs. 18.80 (9.17, 22.13), p<0.001] and leptin/adiponectin ratio (LAR) [6.0 (4.47, 8.42) vs. 2.14 (1.35, 3.42), p<0.001] were significantly higher in PCOS where as adiponectin (ng/ml) was statistically similar between the groups [6.14 (4.36, 9.66) vs. 6.74 (4.54, 8.62), p=0.820]. PCOS group had significantly higher cardiovascular risks than healthy control when they were categorised according to adiponectin/ leptin ratio (p=0.040). Leptin had significant correlation with waist circumference [r= -0.463, p=0.040], waist/hip ratio (WHR) [r= -0.50, p=0025] and waist/height ratio (WHtR) [r= -0.510, p=0.022] where as LAR with WHR [r=-0.617, p=0.004] and WHtR [r= -0.535, p=0.015]. Leptin [AUC=0.916, p<0.001] and LAR [AUC=0.868, p<0.001] were excellent and fair markers of PCOS respectively.
Conclusion: Leptin and LAR may be promising markers of PCOS and cardiovascular risks.
Keywords: Leptin, Adiponectin, Leptin/adiponectin ratio, Polycystic ovary syndrome
Introduction
Polycystic ovary syndrome (PCOS) is a common, complex and heterogeneous reproductive endocrinopathy of females throughout the whole world. Along with its classical reproductive and cutaneous manifestations, the metabolic problems are being increasingly recognized especially during later life.1 The metabolic abnormalities in PCOS are thought to be related with adipose tissue dysfunction. Several adipocytokines secreted from hypertrophied adipocytes are found to be associated with insulin resistance, metabolic syndrome, and cardiovascular complications in PCOS.2 Adiponectin and leptin are two most familiar adipocytokines with opposite relation with obesity and insulin resistance. While adiponectin is usually reduced, leptin is elevated in patients with PCOS. Adiponectin may have anti-inflammatory and insulin sensitizing effects along with promotion of fatty acid oxidation. On the other hand, leptin usually regulates insulin signaling, appetite, reproductive as well as immune function. In comparison to adiponectin, its serum level usually depends on body mass index (BMI).3 Due to their complementary effects, their ratio is thought to be linked with central obesity, insulin resistance, inflammation, atherosclerosis, and cardiovascular risks.4-6 Data regarding the relation of leptin and adiponectin with PCOS and its manifestations are inconclusive. Besides their role as marker of PCOS are not adequately evaluated. The aim of this study was to see the relation of leptin, adiponectin, and their ratio with PCOS and its cardiovascular risks.
Materials and Methods
This case-control study was done in the department of endocrinology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka among 20 newly detected PCOS patients of reproductive age (18-38 years) and similar number of matched control. The study protocol was approved by the institutional review board of BSMMU. Informed written consent was taken from each participant.
PCOS was diagnosed according to revised Rotterdam criteria [two out of three: oligo/anovulation, clinical/ biochemical hyperandrogenemia, polycystic ovarian morphology (PCOM) by ultrasonography (USG) with exclusion of similar diseases].7 Oligomenorrhea was defined as menstrual cycle length >35 days. Clinical hyperandrogenism was defined by modified Ferriman- Gallwey score ≥8 and hyperandrogenemia by free androgen index [FAI= {total testosterone (TT) ÷ sex hormone binding globulin (SHBG)}×100%] >5%. PCOM was diagnosed by ovarian volume >10 ml evaluated by USG during follicular phase of menstrual cycle. Participants with regular menstrual cycle without clinical/ biochemical hyperandrogenism were enrolled as control.
Relevant history (reproductive and family history) were taken, physical examinations [height, weight, waist circumference (WC), hip circumference, blood pressure, hirsutism, acne and acanthosis nigricans] were done and fasting blood was drawn from each participant to measure blood glucose (FBG), insulin, TT, SHBG, luteinizing hormone (LH) follicle stimulating hormone (FSH), lipid profile, adiponectin, and leptin. Oral glucose tolerance test (OGTT) was done.
Glucose was measured by glucose oxidase method, all hormones including SHBG by chemiluminescent immunoassay and lipid by glycerol phosphate dehydrogenase-peroxidase method. Adiponectin measurement was done by two-site sandwich enzyme- linked immunosorbent assay (ELISA) using DRG® Human Adiponectin ELISA (EIA-5838) kit with intra- assay coefficient of variation (CV) of 2.8 – 3.9%. Leptin was measured by DRG leptin (sandwich) ELISA kit- EIA (2395), Inc. USA with intra-assay coefficient of variation of 4.2 – 7.3%. Insulin resistance was measured by homeostasis model assessment of insulin resistance (HOMA-IR). Cardiovascular risk was categorised by adiponectin/leptin ratio (ALR) into normal (ed1), moderate (0.5 to <1) and severe risk (<0.5).4
Statistical analysis was done by SPSS software version 23.0. Data were expressed in mean±(SD), median (interquartile range, IQR) or frequency (percentages, %) depending on their category and distribution. Comparison between groups was done by independent samples t test, Mann Whitney U test, and Pearson’s chi-square/Fisher’s exact test as appropriate. Correlation with leptin, adiponectin and their ratio were done by Spearman’s correlation test. Receiver operating characteristics curve (ROC) analysis was done to see the discriminating index of each of them for PCOS. p values at <0.05 were set as statistically significant.
Results
Data on characteristics of the study population were recorded (table I).
|
Variable categories |
Variables |
PCOS(n= 20) |
Control(n= 20) |
p |
|---|---|---|---|---|
|
Personal history |
Age, years |
27.30±1.29 |
26.45±0.91 |
0.593 |
|
|
Subfertility |
6 (30.0) |
0 (0.0) |
0.020 |
|
|
MR/ abortion |
6 (35.0) |
1 (5.0) |
0.091 |
|
Family history |
PCOS |
6 (30.0) |
0 (0.0) |
0.020 |
|
|
Subfertility |
5 (25.0) |
2 (10.0) |
0.407 |
|
|
Obesity |
13 (65) |
9 (45.0) |
0.341 |
|
|
Hypertension |
10 (50.0) |
13 (65.0) |
0.523 |
|
|
Diabetes mellitus |
14 (70.0) |
11 (55.0) |
0.514 |
|
Physical findings |
BMI, kg/m2 |
29.47±1.35 |
23.71±0.71 |
0.001 |
|
|
Waist circumference, cm |
95.15±2.95 |
80.58±2.06 |
<0.001 |
|
|
Waist/hip ratio |
0.90±0.01 |
0.86±0.01 |
0.002 |
|
|
Waist/height ratio |
0.62±0.02 |
0.52±0.01 |
<0.001 |
|
|
Systolic BP, mm-Hg |
116.00±2.94 |
109.00±2.01 |
0.057 |
|
|
Diastolic BP mm-Hg |
29.47±1.35 |
23.71±0.71 |
0.001 |
|
|
Acne |
12 (60.0) |
2 (10.0) |
0.002 |
|
|
Acanthosis nigricans |
14 (70.0) |
0 (0.0) |
<0.001 |
Independent samples t test and Pearson’s chi-square/Fisher’s exact test were done as appropriate
Personal history of subfertility and family history of PCOS were significantly higher in PCOS group [PCOS vs. control- subfertility and FH of PCOS: 30.0% vs. 0.0%, p=0.020 for both]. All other history were statistically similar between the groups [p=NS for all]. Except systolic blood pressure, PCOS participants had significantly higher value or frequency of all other physical findings [PCOS vs. control- BMI (kg/m2): 29.47±1.35 vs. 23.71±0.71, p=0.001; WC (cm): 95.15±2.95 vs. 80.58±2.06, p<0.001; waist/hip ratio (WHR): 0.90±0.01 vs. 0.86±0.01, p=0.002; waist/height ratio (WHtR): 0.62±0.02 vs. 0.52±0.01, p<0.001; diastolic BP (mm=Hg): 29.47±1.35 vs. 23.71±0.71, p=0.001; acne: 60.0% vs. 10.0%, p=0.002; acanthosis nigricans: 70.0% vs. 0.0%, p<0.001].
The study groups were statistically similar in glycaemic values and triglyceride [p=NS for all]. The PCOS group had significantly different values of other lipid components [PCOS vs. control- TC: 197.0 (177.75, 219.75) vs. 179.0 (159.0, 207.25), p=0.03; LDL-cholesterol: 131.46±8.65 vs. 107.25±7.20, p=0.038;HDL-cholesterol: 48.50 (40.0, 57.75) vs. 42.0 (38.0,46.0), p=0.010, mg/dl]. TT (ng/dl) was significantly higher [50.35 (30.05, 98.15) vs. 19.40 (15.10, 22.07), p<0.001] but SHBG (ng/dl) was significantly lower [9.10 (8.03, 13.10) vs. 19.40 (15.10, 22.07), p<0.001] in PCOS group than the control group. HOMA-IR was also significantly higher in PCOS participants [2.38 (2.07, 5.57) vs. 2.0 (1.36, 2.71), p=0.008] (table II).
|
Variables |
PCOS (n=20) |
Controls (n=20) |
p |
|---|---|---|---|
|
FBG, mmol/L |
5.25±0.16 |
5.23±0.12 |
0.923 |
|
2H-OGTT glucose, mmol/L |
7.75±0.46 |
6.75±0.25 |
0.063 |
|
Triglyceride, mg/dl |
132.65±12.51 |
126.35±14.08 |
0.740 |
|
S. LDL-cholesterol, mg/dl |
131.46±8.65 |
107.25±7.20 |
0.038 |
|
Total cholesterol, mg/dl |
197.0 (177.75, 219.75) |
179.0 (159.0, 207.25) |
0.043 |
|
S. HDL-cholesterol, mg/dl |
48.50 (40.0, 57.75) |
42.0 (38.0, 46.0) |
0.010 |
|
TT, ng/dl |
50.35 (30.05, 98.15) |
19.40 (15.10, 22.07) |
<0.001 |
|
SHBG, ng/dl |
9.10 (8.03, 13.10) |
26.35 (23.95, 65.23) |
<0.001 |
|
HOMA-IR |
2.38 (2.07, 5.57) |
2.0 (1.36, 2.71) |
0.008 |
|
S. adiponectin, ng/ml |
6.14 (4.36, 9.66) |
6.74 (4.54, 8.62) |
0.820 |
|
S. leptin, ng/ml or µg/L |
30.0 (24.15, 68.13) |
18.80 (9.17, 22.13) |
<0.001 |
|
Adiponectin/leptin ratio |
0.17 (0.12, 0.22) |
0.47 (0.29, 0.74) |
<0.001 |
|
Leptin/adiponectin ratio |
6.0 (4.47, 8.42) |
2.14 (1.35, 3.42) |
<0.001 |
Independent samples T test or Mann Whitney U test and was done as appropriate
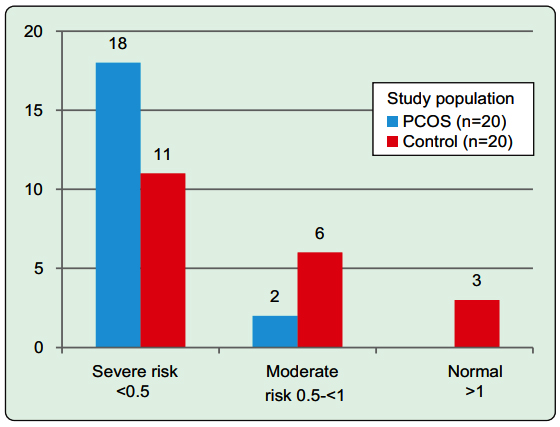
Adiponectin was lower but not statistically different in PCOS patients than control. PCOS group had significantly higher leptin level [30.0 (24.15, 68.13) vs. 18.80 (9.17, 22.13), p<0.001] and lower ALR level [0.17 (0.12, 0.22) vs. 0.47 (0.29, 0.74), p<0.001] than control group. PCOS group had significantly higher cardiovascular risks than healthy control when they are categorized according to ALR (p=0.040) (Figure 1). None of the PCOS patients were in the normal cardiovascular risk group. Among different manifestations, WC [r= -0.463, p= 0.040], WHR [r= - 0.50, p=0.025], and WHtR [r= -0.510, p=0.022] were significantly and negatively correlated with leptin in PCOS. Only WHR [r= -0.617, p=0.004] and WHtR [r= -0.535, p=0.015] were significantly correlated with leptin/adiponectin ratio (LAR) in PCOS patients. Control group had significant correlation of leptin with total cholesterol [r= 0.448, p=0.047] and adiponectin with HDL-cholesterol [r= 0.494, p= 0.027] (table III).
|
Determinants of ‘r’ |
Leptin |
Adiponectin r (p) |
Leptin/adiponectin ratio |
|||
|---|---|---|---|---|---|---|
|
PCOS |
Control |
PCOS |
Control |
PCOS |
Control |
|
|
Age, years |
-0.022 (0.928) |
0.279 (0.233) |
-0.215 (0.363) |
0.136 (0.567) |
0.161 (0.499) |
0.151 (0.526) |
|
BMI, kg/m2 |
-0.421 (0.064) |
-0.165 (0.486) |
0.083 (0.729) |
-0.069 (0.772) |
-0.328 (0.158) |
-0.044 (0.855) |
|
WC, cm |
-0.463 (0.040) |
-0.051 (0.830) |
0.083 (0.728) |
-0.126 (0.597) |
-0.412 (0.071) |
0.0 (1.00) |
|
Waist/hip ratio |
-0.500 (0.025) |
0.187 (0.431) |
0.219 (0.354) |
-0.09 (0.705) |
-0.617 (0.004) |
0.142 (0.550) |
|
Waist/height ratio |
-0.510 (0.022) |
0.012 (0.960) |
0.114 (0.631) |
-0.011 (0.965) |
-0.535 (0.015) |
0.041 (0.865) |
|
Systolic BP, mm-Hg |
-0.024 (0.919) |
-0.036 (0.881) |
0.156 (0.512) |
-0.325 (0.162) |
0.177 (0.455) |
0.102 (0.670) |
|
Diastolic BP, mm-Hg |
-0.021 (0.930) |
-0.041 (0.865) |
0.024 (0.921) |
-0.166 (0.484) |
0.177 (0.455) |
0.005 (0.984) |
|
FBG, mmol/L |
-0.018 (0.941) |
-0.220 (0.351) |
-0.137 (0.566) |
-0.039 (0.870) |
0.141 (0.553) |
-0.192 (0.418) |
|
2H-OGTT glucose, mmol/L |
-0.124 (0.602) |
-0.033 (0.892) |
0.094 (0.693) |
-0.118 (0.620) |
-0.178 (0.454) |
0.286 (0.222) |
|
Triglyceride, mg/dl |
-0.335 (0.149) |
0.033 (0.890) |
0.020 (0.935) |
-0.240 (0.308) |
-0.206 (0.383) |
-0.019 (0.937) |
|
S. LDL-cholesterol, mg/dl |
-0.256 (0.275) |
0.360 (0.119) |
-0.086 (0.719) |
-0.044 (0.855) |
-0.189 (0.424) |
0.364 (0.114) |
|
Total cholesterol, mg/dl |
-0.276 (0.238) |
0.448 (0.047) |
-0.042 (0.860) |
0.029 (0.905) |
-0.148 (0.533) |
0.357 (0.123) |
|
S. HDL-cholesterol, mg/dl |
0.332 (0.153) |
0.102 (0.667) |
0.146 (0.538) |
0.494 (0.027) |
0.329 (0.156) |
-0.024 (0.920) |
|
S. TT, ng/dl |
0.274 (0.243) |
-0.278 (0.235) |
-0.116 (0.627) |
-0.269 (0.251) |
0.337 (0.146) |
0.122 (0.609) |
|
S. LH/FSH ratio |
0.238 (0.313) |
-0.263 (0.262) |
0.272 (0.246) |
-0.180 (0.446) |
-0.131 (0.582) |
-0.024 (0.920) |
|
HOMA-IR |
0.041 (0.863) |
-0.044 (0.855) |
-0.226 (0.339) |
-0.018 (0.940) |
0.247 (0.295) |
-0.017 (0.945) |
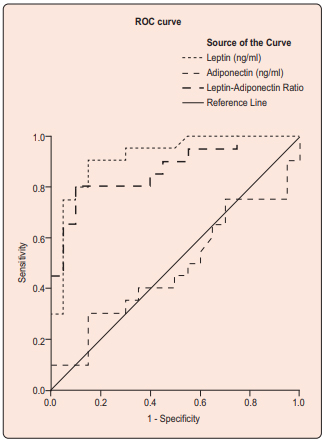
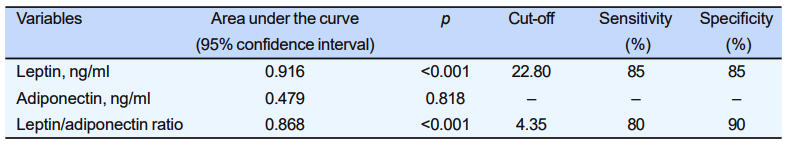
ROC curve analysis showed that leptin had excellent [area under the curve, AUC (95% confidence interval, CI) = 0.916 (0.827, 1.00), p<0.001] and LAR [AUC (95% CI)= 0.868 (0.754, 0.981), p<0.001] had fair (95% CI)= 0.868 (0.754, 0.981), p<0.001] had fair capacity to discriminate PCOS from healthy control. Holding cut-off of 22.80 ng/ml, leptin had both 85% sensitivity and specificity where as with cut-off of 4.35, LAR had 80% sensitivity and 95% specificity in diagnosing PCOS. Adiponectin alone could not be considered marker of PCOS [AUC (95% CI) = 0.479 (0.295, 0.662), p=0.818] (Figure 2). Cardiometabolic risk stratification by adiponectin/leptin ratio Fisher’s exact test was done
Discussion
This study was done among 20 reproductive age PCOS patients and equal number of matched healthy control to see the relation of leptin, adiponectin and their ratio with PCOS and its cardiovascular risks. PCOS is associated with obesity and adipose tissue dysfunction resulting in altered production of different adipokines including adiponectin and leptin. Although the adiponectin in PCOS was lower it was not statistically different from healthy control in our study. This finding is also observed by others especially when adiponectin is adjusted for BMI.8-11 In contrast, meta-analysis found lower level of adiponectin in PCOS.12 On the other hand, leptin was significantly higher in PCOS participants. Our finding is consistent with other studies.13,14 We also found significantly lower ALR or higher LAR in PCOS group than control. This relation was also reported by other studies. 15-17
In this study, the PCOS group had several cardio- vascular risk factors in comparison to the healthy control which was also revealed when we categorize the study population by ALR. Adipocytokines mediate the obesity associated cardiovascular risks.18 Correlation between adiponectin and leptin with visceral adiposity along with metabolic syndrome was found in PCOS.19 So, they may mediate the pathogenesis of cardiovascular complications in PCOS.
We found significant correlation of leptin and LAR with WHtR rather than BMI in PCOS group. Studies also showed better usefulness of WHtR than BMI in predicting PCOS, insulin resistance as well as cardiovascular diseases.20,21 Obirikorang et al also found correlation of WHtR with leptin and LAR.22 WC and WHR ratios are established cardiovascular risk factors. Their associations with PCOS and adipocytokines in this study also indicate their relation with cardiovascular diseases. We did not find any correlation of the adipocytokines with androgen which was similar to other studies.23 Lecke et al also reported that adipocytokines’ altered secretion was independent of free androgen and BMI.24 We also did not find any correlation of HOMA-IR with our investigated adipocytokines that was similar to some studies.9,25,26 Fat mass rather than insulin resistance might be the predominant determinant of adipocytokines production in PCOS.
We observed leptin and LAR as markers of PCOS with high sensitivity and specificity. The high discriminating index of our studied adipokines and their ratio was similar to several studies with different discriminating indices and superiority of one over another.16,17,26 These markers can serve as both diagnostic as well as cardiovascular risk stratification in PCOS. However, they found LAR better than individual value of leptin or adiponectin.
One of the limitations of this study was it’s small sample size. We could not measure the high molecular weight adiponectin which may better correlate with insulin resistance and glucose intolerance.
Conclusion
PCOS is characterised by higher leptin and LAR. Their correlations with WHR and WHtR also indicate their associations with cardiometabolic risk factors. Besides, they seemed to be novel and powerful markers of PCOS. Larger sample size with prospective studies is needed to reveal their utility especially in clinical practice.
Acknowledgments
We are grateful to our patients along with the department of biochemistry and department of Microbiology, BSMMU for their technical support. Financial support by Research & Development of BSMMU as well as the PCOS Study Group, Endocrinology department, BSMMU are duly acknowledged.
References
- De Melo AS, Dias SV, De Carvalho Cavalli R, Cardoso VC, Bettiol H, Barbieri MA, et al. Pathogenesis of polycystic ovary syndrome: Multifactorial assessment from the foetal stage to menopause. Reproduction. 2015;150:R11-24.
Doi:10.1530/REP-14-0499. - Delitala AP, Capobianco G, Delitala G, Cherchi PL, Dessole S. Polycystic ovary syndrome, adipose tissue and metabolic syndrome. Archives of Gynecology and Obstetrics. 2017;296:405-19.
Doi:10.1007/s00404-017-4429-2 - Villa J, Pratley RE. Adipose tissue dysfunction in polycystic ovary syndrome. Current Diabetes Reports. 2011;11:179-84.
Doi:10.1007/s11892-011-0189-8. - Frühbeck G, Catalán V, Rodríguez A, Ramírez B, Becerril S, Salvador J, et al. Adiponectin-leptin ratio is a functional biomarker of adipose tissue inflammation. Nutrients. 2019;1192:454.
Doi:10.3390/nu11020454 - Satoh N, Naruse M, Usui T, Tagami T, Suganami T, Yamada K, et al. Leptin-to-adiponectin ratio as a potential atherogenic index in obese type 2 diabetic patients. Diabetes Care. 2004;27:2488-90.
Doi:10.2337/diacare.27.10.2488. - Rahmani A, Toloueitabar Y, Mohsenzadeh Y, Hemmati R, Sayehmiri K, Asadollahi K. Association between plasma leptin/adiponectin ratios with the extent and severity of coronary artery disease. BMC Cardiovasc Disord. 2020;20:474.
Doi:10.1186/s12872-020-01723-7. - Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25.
Doi:10.1016/j.fertnstert.2003.10.004. - Spranger J, Möhlig M, Wegewitz U, Ristow M, Pfeiffer AFH, Schillt T, et al. Adiponectin Is Independently Associated With Insulin Sensitivity in Women With Polycystic Ovary Syndrome. Obstet Gynecol Surv. 2005;12:129-34.
Doi:10.1111/j.1365-2265.2004.02159.x. - Orio F, Palomba S, Cascella T, Milan G, Mioni R, Pagano C, et al. Adiponectin levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2619-23.
Doi:10.1210/jc.2002-022033. - Panidis D, Kourtis A, Farmakiotis D, Mouselech T, Rousso D, Koliakos G. Serum adiponectin levels in women with polycystic ovary syndrome. Hum Reprod. 2003;18:1790-6.
Doi:10.1093/humrep/deg353. - Gözüküçük M, Yarcl Gürsoy A, Destegül E, Taºkln S, Satlroçlu H. Adiponectin and leptin levels in normal weight women with polycystic ovary syndrome. Horm Mol Biol Clin Investig. 2020;41.
Doi:10.1515/hmbci-2020-0016. - Li S, Huang X, Zhong H, Peng Q, Chen S, Xie Y, et al. Low circulating adiponectin levels in women with polycystic ovary syndrome: An updated meta-analysis. Tumor Biology. 2014;35:3961-73.
Doi:10.1007/s13277-013-1595-0. - Chakrabarti J. Serum leptin level in women with polycystic ovary syndrome: Correlation with adiposity, insulin, and circulating testosterone. Ann Med Health Sci Res. 2013;3:191-6.
Doi:10.4103/2141-9248.113660. - Jalilian N, Haghnazari L, Rasolinia S. Leptin and body mass index in polycystic ovary syndrome. Indian J Endocrinol Metab. 2016;20:324-8.
Available from:www.ijem.in/text.asp?2016/20/3/324/180005 - Chen CI, Hsu MI, Lin SH, Chang YCI, Hsu C Sen, Tzeng CR. Adiponectin and leptin in overweight/obese and lean women with polycystic ovary syndrome. Gynecol Endocrinol. 2015;31:364-8.
Doi:10.3109/09513590.2014.984676. - Sarray S, Madan S, Saleh LR, Mahmoud N, Almawi WY. Validity of adiponectin-to-leptin and adiponectin-to-resistin ratios as predictors of polycystic ovary syndrome. Fertil Steril. 2015;104:460-6.
Doi:10.1016/j.fertnstert.2015.05.007. - Golbahar J, Das NM, Al-Ayadhi MA, Gumaa K. Leptin-to- adiponectin, adiponectin-to-leptin ratios, and insulin are specific and sensitive markers associated with polycystic ovary syndrome: A case-control study from Bahrain. Metab Syndr Relat Disord. 2012;10:98-102.
Doi:10.1089/met.2011.0075. - Landecho MF, Tuero C, Valentí V, Bilbao I, de la Higuera M, Frühbeck G. Relevance of leptin and other adipokines in obesity-associated cardiovascular risk. Nutrients. 2019;11:2664.
Doi:10.3390/nu11112664. - Glintborg D, Andersen M, Hagen C, Frystyk J, Hulstrøm V, Flyvbjerg A, et al. Evaluation of metabolic risk markers in polycystic ovary syndrome (PCOS). Adiponectin, ghrelin, leptin and body composition in hirsute PCOS patients and controls. Eur J Endocrinol. 2006;155:337-45.
Doi:10.1530/ eje.1.02207. - Bhattacharya K, Sengupta P, Dutta S, Chaudhuri P, Das Mukhopadhyay L, Syamal AK. Waist-to-height ratio and BMI as predictive markers for insulin resistance in women with PCOS in Kolkata, India. Endocrine. 2021;72:86-95.
Doi:10.1007/s12020-020-02555-3. - Sabah KMDN, Chowdhury AW, Khan HLR, Hasan ATMH, Haque S, Ali S, et al. Body mass index and waist/height ratio for prediction of severity of coronary artery disease. BMC Res Notes. 2014;7:246.
Doi:10.1186/1756-0500-7-246. - Obirikorang C, Owiredu WKBA, Adu-Afram S, Acheampong E, Asamoah EA, Antwi-Boasiakoh EK, et al. Assessing the variability and predictability of adipokines (adiponectin, leptin, resistin and their ratios) in non-obese and obese women with anovulatory polycystic ovary syndrome. BMC Res Notes. 2019;12:513.
Doi:10.1186/s13104-019-4546-z. - Baldani DP, Skrgatic L, Kasum M, Zlopasa G, Kralik Oguic S, Herman M. Altered leptin, adiponectin, resistin and ghrelin secretion may represent an intrinsic polycystic ovary syndrome abnormality. Gynecol Endocrinol. 2019;35:401-5.
Doi: - Lecke SB, Mattei F, Morsch DM, Spritzer PM. Abdominal subcutaneous fat gene expression and circulating levels of leptin and adiponectin in polycystic ovary syndrome. Fertil Steril. 2011;95:2044-49.
- Erturk E, Kuru N, Savci V, Tuncel E, Ersoy C, Imamoglu S. Serum leptin levels correlate with obesity parameters but not with hyperinsulinism in women with polycystic ovary syndrome. Fertil Steril. 2004;82:1364-68.
- Olszanecka-Glinianowicz M, Kuglin D, Dbkowska-Huæ A, Ska³ba P. Serum adiponectin and resistin in relation to insulin resistance and markers of hyperandrogenism in lean and obese women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2011;154:51-56.
Department of Endocrinology, Banagabandhu Sheikh Mujib Medical University, Shahbagh, Dhaka-1000, Bangladesh.
hasanatdr@yahoo.com
 0000-0001-8151-9792
0000-0001-8151-9792
Submission
24 May 2021
Accepted
17 October 2021
Published
01 December 2021
Apply citation style format of Bangladesh Medical Research Council
Issue
Vol 47 No 3 (2021)
Section
Research Articles
Ethical Clearance
Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
Financial Support
Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
Conflict of Interest
There is no conflict of interest.