Roksana Parvin
Department of General Paediatrics, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh.
Suraiya Begum
Department of Paediatrics, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh.
Abul Basher Md. Osman Hayder Mazumder
Department of General Paediatrics, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh.
Ismat Jahan
Department of General Paediatrics, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh.
Kohinoor Jahan Shyamaly
Department of Paediatrics, District Hospital Sherpur, Bangladesh.
Keywords: Obesity, Insulin resistance, HOMA-IR, Puberty.
DOI: 10.3329/bmrcb.v47i2.57782
Abstract
Background: Insulin resistance has evolved as an important metabolic alteration in obese children especially during adolescence. Emerging data suggest that insulin resistance in obesity play the key role in the pathogenesis of hypertension, dyslipidemia, impaired glucose tolerance, type 2 diabetes mellitus, non-alcoholic fatty liver disease and metabolic syndrome.
Objective: The aim of this study was to assess the insulin resistance status of Bangladeshi obese adolescents in hospital settings.
Methods: It was a cross sectional study done in children, aged 10 to 16 years, attending the paediatric endocrine clinic, paediatric outpatient and paediatric inpatient department, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka over a period of 15 months. Homeostasis Model Assessment of Insulin Resistance (HOMA- IR) value was obtained from fasting insulin & fasting blood glucose level to assess insulin resistance in obese and normal weight children.
Results: Among obese adolescents, 78.8% were insulin resistant in comparison to16% in non-obese adolescent. Mean HOMA-IR level of obese group were significantly higher than non-obese group (4.46±1.82 Vs 1.98±1.26, p<0.001). Insulin resistance was observed in 73.8% and 84.2% among grade 1 obese and grade 2 obese adolescents respectively. HOMA-IR showed positive correlation with BMI and waist circumference. Insulin resistance showed significant association with central obesity, puberty and acanthosis nigricans (AN).
Conclusion: In this study, insulin resistance was observed in higher frequency among obese adolescents than normal weight adolescents. Obese adolescents with central obesity, acanthosis nigricans and puberty are at increased risk of insulin resistance.
Keywords: Obesity, Insulin resistance, HOMA-IR, Puberty.
Introduction
Obesity in children and adolescent is rising alarmingly and approaching an epidemic proportion in many countries.1 World Health Organization (WHO) considered childhood obesity as one of the most serious public heath challenges in 21st century.2 A systematic analysis of 1,769 reports representing 188 countries revealed that the worldwide prevalence of childhood overweight and obesity rose by nearly 50% over a span of three decades.3 In Bangladesh, the prevalence of obesity was found to be 3.5% among school aged children.4
In recent years, studies have shown that type 2 diabetes mellitus has increased dramatically in children and adolescents throughout the world.5 This increase in frequency of type 2 diabetes seems to parallel the increase in prevalence and severity of obesity in children and adolescents.6 Insulin resistance is considered a pre-diabetic condition characterised by imbalance of glucose metabolism, causing an increase in insulin production and a given amount of insulin producing a subnormal biological response.7
Adipose tissue seems to play a key role in the pathogenesis of insulin resistance through several released metabolites, hormones and adipocytokines that can affect different steps in insulin action.8 Excessive accumulation of body fat, especially abdominal fat, is associated with increased levels of free fatty acids in the bloodstream, which in turn inhibit carbohydrate metabolism via substrate competition and impaired insulin signaling by lowering receptor sensitivity in cell membranes, creating the insulin resistance.9 Several Studies have emphasised that children with insulin resistance have a higher predisposition to the future development of hypertension, dyslipidemia, metabolic syndrome, type II diabetes mellitus and cardiovascular disease.10-12 Longitudinal Study also observed that cardiovascular risk factors dyslipidemia and hypertension decreased in obese children with any degree of decrease in insulin resistance, independently of changes in body weight.13
In adolescents, insulin sensitivity declines considerably during pubertal period of life. Multiple cross sectional studies showed the association of puberty and declined insulin sensitivity in adolescents.12,14 Longitudinal study also revealed that insulin sensitivity undergoes a decline of around 25–50% during puberty.15
There is different method capable of estimating the degree of sensitivity of individual to insulin. The homeostatic model assessment method for insulin resistance (HOMA-IR) index is a widely used method in adults and has been validated in children and adolescents as an indicator of insulin resistance.16 HOMA-IR index increases with the increase in insulin resistance in individual. There is paucity of data in Bangladesh regarding insulin resistance in obese adolescent. Aim of this study was to assess insulin resistance in obese adolescents in a tertiary care hospital in Bangladesh.
Materials and Methods
It was a cross sectional study conducted in Department of Paediatrics (Paediatric Endocrinology clinic, paediatric outpatient and inpatient department), Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh from March 2018 to July 2019. A total of 80 obese adolescents aged 10 to 16 years who attended hospital for evaluation of obesity were included in the study. Age and sex matched 50 adolescents without obesity or overweight were included in the study as control. The weight was measured by using electronic weighing machine, height was measured by stadiometer and waist Circumference (WC) was measured midway between the lowest rib and the superior border of the iliac crest.
To assess obesity, BMI was categorised for particular age and sex according to Centers for disease control and prevention (CDC) criteria. Here, children having BMI for age and sex 5th to 85th percentile of reference population were classified as nonobese and children having BMI for age and sex ≥95th percentile of reference population were classified as obese. Obese group were further subdivided into 2 groups; grade1 obese and grade 2 obese (severe obese) group. Grade 1 obese were defined as adolescents having BMI e” 95th percentile and <120% of 95th centile and grade II obese (Severe obese) were defined as adolescents having BMI ≥120% of 95th centile for age and sex, according to CDC growth chart 2000 or an absolute BMI ≥35 kg/m2, whichever is lower based.17 Central obesity or raised waist circumference was defined as waist circumference ³90th centile for age and sex, according to CDC (Anthropometric Reference Data for Children and Adults: United States), 2007–2010. Acanthosis nigricans and pubertal status of individual according to Tanner were assessed. Adolescents in tanner stage 1 were classified as prepubertal, adolescents in tanner stage 2,3,4 were classified as pubertal. Children who were taking systemic steroid for any cause or on any intervention for obesity like dietary restriction or taking any drug for impaired glucose homeostasis or suffering from genetic, endocrine (eg. Hypothyroidism, Cushing Syndrome, Hypothalamic disorder) and any neurological disease (Epilepsy getting Sodium valproate) that might lead to overweight or obesity were excluded from this study. We also excluded children having any known pancreatic disease or liver disease and children who were in pubertal stage 5 (postpubertal stage). The study protocol was approved by Institutional Review Board of BSMMU, Dhaka.
After considering inclusion and exclusion criteria, fasting blood glucose and fasting insulin level were measured in study participants. Blood glucose was assayed by hexokinase method and insulin levels was measured by chemiluminescent microparticle immunoassay method. HOMA-IR index was obtained by calculating the product of fasting insulin (microU/ ml) and fasting blood glucose (mmol/L) divided by 22.5.18 HOMA-IR index was used to evaluate insulin resistance. Study subjects were defined as insulin resistant when HOMA-IR values were ≥3 and insulin sensitive when HOMA-IR values were <3.19
Statistical analysis was performed by using SPSS Qualitative data were analysed by chi-square test, quantitative data were analyzed by unpaired t test and to evaluate correlation between two variables pearson correlation test and scattered diagram were applied. p value d”0.05 with 95.0% confidence interval was considered as the level of statistical significance.
Results
The study included 80 obese and 50 non-obese adolescents aged 10 to 16 years. Male were 49.23% and female were 50.76%. Mean age was about 11 years in both group, and age and sex of obese and non-obese adolescents showed no significant difference. Mean BMI was significantly higher in obese than non-obese adolescents (p<0.001) and waist circumference e” 90th centile was more in obese group than nonobese group (66.3% vs 0%, p<0.001). Seventy percent of obese and 2% of non-obese adolescents had acanthosis nigricans, and it was significant (p<0.001). Most of the adolescents in both groups already entered puberty and no significant difference was found between two groups. (table I)
Clinical characteristics |
Obese (n=80) |
Non obese (n=50) |
p-value |
|---|---|---|---|
Age (Years) Mean ±SD |
11.84±1.63 |
11.90±1.17 |
0.832 |
Sex n(%) |
|
|
|
Male |
42(52.5%) |
24(48%) |
0.618 |
Female |
38(47.5%) |
26(52%) |
|
BMI (kg/m2) Mean±SD |
28.70±4.02 |
18.46±1.62 |
<0.001 |
Waist Circumference n(%) |
|
|
|
e”90th centile |
55(68.75%) |
0(0%) |
<0.001 |
<90th centile |
25(31.25%) |
50(100%) |
|
Acanthosis Nigricans n(%) |
|
|
|
Present |
56(70.0%) |
1(2.0%) |
<0.001 |
Absent |
24(30.0%) |
49(98.0%) |
|
Pubertal status n(%) |
|
|
|
Prepubertal |
22(27.5%) |
16(32.0%) |
0.583 |
Pubertal |
58(72.5%) |
34(68%) |
|
Among obese adolescents 78.8% were insulin resistant where as 16% non-obese adolescents were insulin resistant (p=<0.001) and mean HOMA-IR value was significantly higher in obese than non-obese group (4.46±1.83 vs 1.98±1.26, p <0.001) (table II).
|
Obese n=80 |
Non-obese n=50 |
P-value |
|---|---|---|---|
Insulin resistance statusn(%) |
|
|
|
Insulin resistant |
63(78.8%) |
8(16.0%) |
<0.001 |
Insulin sensitive |
17(21.3%) |
42(84.0%) |
|
HOMA-IR value (mean ±SD ) |
4.46±1.82 |
1.98±1.26 |
<0.001 |
Mean HOMA-IR level of grade II obese (severe obese) group was significantly higher than grade I obese group (5.06±1.89 VS 3.92±1.58, P<0.005). In 73.8% grade I obese adolescents and in 84.2% grade II obese adolescents had insulin resistant but on statistical analysis there was no significant difference (p=0.256) (table III).
|
Grade I Obese n= 42 |
Grade II Obese n= 38 |
p-value |
|---|---|---|---|
Insulin resistance status |
|
|
|
Insulin resistant |
31(73.8%) |
32(84.2%) |
0.256 |
Insulin sensitive |
11(26.2%) |
6(15.8%) |
|
HOMA-IR value |
|
|
|
Mean ±SD |
3.92±1.58 |
5.06±1.89 |
0.005 |
In insulin resistant obese adolescent 89% had acanthosis nigricans, 81% were in puberty and 75% had central obesity. On Chi-square analysis acanthosis nigricans (p<0.001), central obesity (p<0.03) and puberty (p=0.006) were significantly associated with insulin resistance in obese adolescents (table IV).
|
Insulin resistant, n=63 |
Insulin sensitive, n=17 |
p value |
|---|---|---|---|
Acanthosis nigricans |
|
|
|
Present |
56 (89%) |
0(0%) |
<0.001 |
Absent |
7(11%) |
17(100%) |
|
Pubertal stage |
|
|
|
Pubertal |
51(81%) |
7(41%) |
0.006 |
Prepubertal |
12(19%) |
10(59%) |
|
Central obesity |
|
|
|
Present |
47(75%) |
8(47%) |
<0.03 |
Absent |
16(25%) |
9(53%) |
|
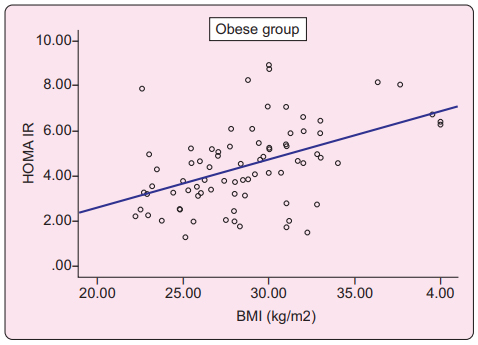
Scatter diagram showed significant positive correlation of body mass index (BMI) with HOMA-IR (p=<0.001) as HOMA-IR value was increased with increasing value of BMI in obese adolescents (Figure 1).
Discussion
Insulin resistance is thought to be the key morbidity, which acts as a link between obesity and metabolic and cardiovascular diseases.20 Obesity causes insulin resistance by a number of mechanisms, including impaired insulin signaling, defects in glucose transport, defective insulin clearance and elevated intra portal free fatty acids with increased systemic adipocyte cytokines.21
The exact prevalence of insulin resistance is not well established in adolescents. Prevalence of insulin resistance in obese children and adolescents vary greatly in different studies depending on different age group, ethnicity, methods used for determination of insulin resistance and also use of different definition of insulin resistance.22-24
In present study, frequency of insulin resistance and mean HOMA-IR value was significantly higher in obese group in comparison to non-obese adolescents. In obese group, 78% adolescents were insulin resistant. These findings of our study match with the results of recent studies. Singh et al. found that prevalence of IR was 77.0% in obese adolescents and 19.7% in non-obese adolescents in their study.23 In another study done on US adolescents also revealed that mean HOMA-IR value was significantly higher in obese adolescents than non-obese adolescent (4.93 vs. 2.30) and prevalence of insulin resistance was 52.1% in obese adolescents.22
In this study mean HOMA-IR value of grade 2 obese adolescents were significantly higher than grade 1 obese adolescents (p=0.005). No significant difference was found when frequency of insulin resistance was compared between grade 1 and grade 2 obese adolescents (73.8% vs 84.2%, p=0.256). Scatter diagram and Pearson correlation efficient test showed positive correlation of BMI with HOMA-IR level. Other recent studies done in different countries observed almost similar findings in their study where HOMA-IR value increased with increasing BMI.23,25,26
In pathogenesis of insulin resistance, fat distribution in body is more important than the total body fat. Visceral fat has a better correlation with insulin resistance than s.c. or total body fat.27 Excessive fatty-acid release from abdominal visceral adipose tissue into the portal circulation has an important inûuence on hepatic metabolism and islet cell function.28 Visceral fat also secrete higher levels of inûammatory adipokines than subcutaneous fat, which hamper insulin action.29 However. BMI value can not indicate body fat distribution and magnitude of visceral adiposity. Waist circumference (WC) are the measurements most commonly used to estimate abdominal fat because they have a positive, significant correlation to the amount of intra-abdominal fat as assessed by imaging studies both in adults and children.30 Our study finding also support the role of visceral adiposity in insulin resistance. Among obese group, waist circumference ≥90th percentile was significantly associated with insulin resistance (p<0.03).This finding were similar to the findings observed by other studies.12,23,25,26
In this study, we found significant association of acanthosis nigricans with insulin resistance in obese adolescents(p<0.001) which was consistent with the result of other studies done by Li et al. and Yamazaki et al.31,32 Acanthosis nigricans is a skin disorder which has been formally established as a clinical marker of insulin resistance in type 2 diabetes by American diabetic association.33 The theory is that high concentrations of insulin can stimulate epidermal keratinocyte and dermal fibroblast proliferation through high affinity binding to insulin-like growth factor 1 (IGF-1) receptors and elevated IGF-1 levels in obese patients.34
Although the phenomenon of pubertal insulin resistance is well documented, the mechanism has not been clearly determined. However, some studies have hypothesized that the effect may be due to rapid acceleration of body fat during puberty.35,36 Alternatively, it has been hypothesized that the fall in insulin sensitivity may be driven by elevated level of sex hormones and transient changes in growth hormone levels in puberty. However insulin sensitivity again increases after puberty where sex hormones remain elevated in postpubertal period.37 In our study, among pubertal obese patients 87.93% were insulin resistant, which was significantly higher (p=0.006) in comparison to prepubertal obese subjects (54%). These findings of our study were comparable to result of the study done by Romualdo et al and Kurtoglu et al.12,14 The present study has certain limitations. It was a cross sectional, hospital based study and family history of insulin resistance or diabetes mellitus was not evaluated in this study.
Present study showed high prevalence of insulin resistance in obese adolescents and higher BMI is associated with higher HOMA-IR value. Insulin resistance is associated with metabolic and cardiovascular complication suggested by mutiple studies. So, prevention, timely diagnosis and appropriate management of obesity as well as insulin resistance is important to reduce the associated risk of metabolic and cardiovascular diseases in young population.
Conclusion
In this study, prevalence of insulin resistance in obese adolescents was 78.8%. Mean HOMA-IR level was significantly higher in obese adolescents. Insulin resistance in obese adolescents were associated with central obesity, acanthosis nigricans and puberty. Though high frequency of insulin resistance was found in both grade I and grade II obese adolescents, HOMA- IR level were significantly higher in grade II obese adolescents. However, further study with large sample size is suggested to confirm the findings.
Acknowledgments
We acknowledge with gratitude the financial support of the Research Grant Committee of BSMMU, Dhaka. We also acknowledge the financial assistance provided by Bangladesh Medical Research Council (BMRC), Dhaka, Bangladesh for the study. We are thankful to all participants for their cooperation and active participations. We would like to express our appreciation towards the BSMMU authority for their administrative support.
References
- Wang Y, Lobstein TI. Worldwide trends in childhood overweight and obesity. International journal of pediatric obesity. 2006; 1:11-25.
DOI: 10.1080/17477160600586747 - World health organization. Childhood overweight and obesity:57th world health assembly, Geneva, 2004.
DOI: 10.1787/a47d0cd2-en - Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980– 2013: a systematic analysis for the Global Burden of Disease Study 2013. The lancet. 2014; 384:766-81.
DOI: 10.1016/S0140-6736(14)60460-8 - Bulbul T, Hoque M. Prevalence of childhood obesity and overweight in Bangladesh: findings from a countrywide epidemiological study. BMC Pediatrics. 2014;14:1-8.
DOI: 10.1186/1471-2431-14-86 - Pulgaron ER, Delamater AM. Obesity and type 2 diabetes in children: epidemiology and treatment. Current diabetes reports. 2014; 14:508.
DOI: 10.1007/s11892-014-0508-y - Arslanian SA. Type 2 Diabetes Mellitus in Children: Pathophysiology and Risk Factors: Division of Pediatric Endocrinology, Metabolism and Diabetes Mellitus Children’s Hospital of Pittsburgh, Pennsylvania, USA. Journal of Pediatric Endocrinology and Metabolism. 2000;13 (Supplement): 1385-94.
DOI: 10.1515/jpem-2000-s612 - Matthaei S, Stumvoll M, Kellerer M, Haring HU. Pathophysiology and pharmacological treatment of insulin resistance. Endocrine Reviews. 2000; 21:585-618
DOI: 10.1210/edrv.21.6.0413 - Matsuzawa Y. White adipose tissue and cardiovascular disease. Best practice & research Clinical Endocrinology & Metabolism. 2005;19:637-47.
DOI: 10.1016/j.beem.2005.07.001 - Ferrier DR. Biochemistry. Philadelphia:Lippincott Williams & Wilkins; 2014. P.622-628.
DOI: - Ferreira AP, Oliveira CE, França NM. Metabolic syndrome and risk factors for cardiovascular disease in obese children: the relationship with insulin resistance (HOMA-IR). Jornal de Pediatria. 2007; 83:21-6.
DOI: 10.2223/jped.1562 - Medeiros CC, Ramos AT, Cardoso MA, França IS, Cardoso AD, Gonzaga NC. Insulin resistance and its association with metabolic syndrome components. Arquivos Brasileiros de Cardiologia. 2011;97:380-9.
DOI: 10.1590/s0066-782x2011005000089 - Romualdo MC, Nóbrega FJ, Escrivão MA. Insulin resistance in obese children and adolescents. Jornal de Pediatria. 2014; 90:600-7.
DOI: 10.1016/j.jped.2014.03.005 - Reinehr T, De Sousa G, Andler W. Longitudinal analyses among overweight, insulin resistance, and cardiovascular risk factors in children. Obesity Research. 2005; 13:1824-33.
DOI: 10.1038/oby.2005.222 - Kurtoðlu S, Hatipoðlu N, Mazýcýoðlu M, Kendirici M, Keskin M, Kondolot M. Insulin resistance in obese children and adolescents: HOMA” IR cut” off levels in the prepubertal and pubertal periods. Journal of Clinical Research in Pediatric Endocrinology. 2010; 2:100.
DOI: 10.4274/jcrpe.v2i3.100 - Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001; 50:2444-50.
DOI: 10.2337/diabetes.50.11.2444 - Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2000; 115:e500-3.
DOI: 10.1542/peds.2004-1921 - Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128:1689- 1712.
DOI: 10.1161/cir.0b013e3182a5cfb3 - Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and â-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412-9.
DOI: 10.1007/bf00280883 - Yin J, Li M, Xu L, Wang Y, Cheng H, Zhao X, Mi J. Insulin resistance determined by Homeostasis Model Assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers. Diabetology & Metabolic Syndrome. 2013; 5:1-9.
DOI: 10.1186/1758-5996-5-71 - Chiarelli F, Marcovecchio ML. Insulin resistance and obesity in childhood. European Journal of Endocrinology. 2008; 159(suppl_1):S67-74.
DOI: 10.15 30/eje-08-0245 - Kahn BB, Flier JS. Obesity and insulin resistance. The Journal of Clinical Investigation. 2000;10:473-81.
DOI: 10.1172/jci10842 - Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among US adolescents: a population-based study. Diabetes Care. 2006; 29:2427-32.
DOI: 10.2337/dc06-0709 - Singh Y, Garg MK, Tandon N, Marwaha RK. A study of insulin resistance by HOMA-IR and its cut-off value to identify metabolic syndrome in urban Indian adolescents. Journal of Clinical Research in Pediatric Endocrinology. 2013;5:245.
DOI: 10.1515/jpem-2013-0020 - Tresaco B, Bueno G, Moreno LA, Garagorri JM, Bueno M. Insulin resistance and impaired glucose tolerance in obese children and adolescents. Journal of Physiology and Biochemistry. 2003; 59:217-23.
DOI: 10.1007/bf03179918 - Gobato AO, Vasques AC, Zambon MP, Barros Filho AD, Hessel G. Metabolic syndrome and insulin resistance in obese adolescents. Revista Paulista de Pediatria. 2014; 32:55-9.
DOI: 10.1590/s0103-05822014000100010 - Rocco ER, Mory DB, Bergamin CS, Valente F, Miranda VL, Calegare BF, Silva RQ, Dib SA. Optimal cutoff points for body mass index, waist circumference and HOMA-IR to identify a cluster of cardiometabolic abnormalities in normal glucose-tolerant Brazilian children and adolescents. Arquivos Brasileiros de Endocrinologia & Metabologia. 2011; 55:638-45.
DOI: 10.1590/s0004-27302011000800020 - Caprio SO, Hyman LD, Limb CH, McCarthy SH, Lange RO, Sherwin RS, Shulman GE, Tamborlane WV. Central adiposity and its metabolic correlates in obese adolescent girls. American Journal of Physiology-Endocrinology and Metabolism. 1995; 269:E118-26.
DOI: 10.1152/ajpendo.1995.269.1.e118 - Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. The Lancet. 2005; 365:1333-1346.
DOI: 10.1016/s0140-6736(05)61032-x - Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273-82.
DOI: 10.1210/en.2003-1336 - World Health Organization. Waist circumference and waist- hip ratio: report of a WHO expert consultation, Geneva, 8-11 December 2008.
DOI: - Li HJ, Huang CY, Lee HC, Chen MR, Lo FS, Lin CH, Wang AM, Shih BF, Lee YJ. Insulin resistance in obese adolescents. Acta Paediatrica Taiwanica= Taiwan er ke yi xue hui za zhi. 2005;46:61-6.
DOI: - Yamazaki H, Ito S, Yoshida H. Acanthosis nigricans is a reliable cutaneous marker of insulin resistance in obese Japanese children. Pediatrics international. 2003;45: 701-5.
DOI: 10.1111/j.1442-200x.2003.01812.x - Sinha S, Schwartz RA. Juvenile acanthosis nigricans. Journal of the American Academy of Dermatology. 2007;57:502-8.
DOI: 10.1016/j.jaad.2006.08.016 - Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatology online journal. 2008;14
DOI: 10.1210/er.2010-0020 - Caprio S, Plewe G, Diamond MP, Simonson DC, Boulware SD, Sherwin RS, Tamborlane WV. Increased insulin secretion in puberty: a compensatory response to reductions in insulin sensitivity. The Journal of Pediatrics. 1989;114:963-7.
DOI: 10.1016/s0022-3476(89)80438-x - Travers SH, Jeffers BW, Eckel RH. Insulin resistance during puberty and future fat accumulation. The Journal of Clinical Endocrinology & Metabolism. 2002; 87:3814-8.
DOI: 10.1210/jcem.87.8.8765 - American Diabetes Association. Type 2 diabetes in children and adolescents. Pediatrics. 2000;105:671-80.
DOI: 10.1542/peds.105.3.671
Department of General Paediatrics, Bangabandhu Sheikh Mujib Medical University, Shahbagh, Dhaka-1000, Bangladesh.
roksana2parvin@gmail.com
 0000-0003-4552-8418
0000-0003-4552-8418
Submission
15 March 2021
Accepted
30 June 2021
Published
01 August 2021
Apply citation style format of Bangladesh Medical Research Council
Issue
Vol 47 No 2 (2021)
Section
Research Articles
Ethical Clearance
Institutional Review Board of Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bnagladesh.
Financial Support
BSMMU and Bangladesh Medical Research Council (BMRC), Dhaka, Bangladesh.
Conflict of Interest
There was no conflict of interest.