Majidul Islam
University of Rajshahi, Rajshahi, Bangladesh.
Rumana Yesmin Hasi
University of Rajshahi, Rajshahi, Bangladesh.
Hanif Ali
University of Rajshahi, Rajshahi, Bangladesh.
Polash Chandra Karmakar
University of Rajshahi, Rajshahi, Bangladesh.
Rowshanul Habib
University of Rajshahi, Rajshahi, Bangladesh.
Mohammed A. Satter
Bangladesh Council of Scientific and Industrial Research (BCSIR), Dr. Qudrat-E-Khuda Road, Dhaka, Bangladesh.
Tanzima Yeasmin
University of Rajshahi, Rajshahi, Bangladesh.
Keywords: Hibiscus sabdariffa, Antioxidant, Anticancer, Apoptosis
DOI: 10.3329/bmrcb.v47i2.57774
Abstract
Background: Since chemical anticancer drugs are found to have adverse effect on body, nowadays people are motivated towards natural agents to treat cancer. Fruits and vegetables are potential source of natural anticancer agents that protect our body against cancer by scavenging and neutralizing free radicals.
Objective: The study was aimed to explore antioxidant and anticancer properties of methanolic extract of leaves of Hibiscus sabdariffa L. (MEHSL).
Methods: In vitro assays were used to examine the different types of phytochemical content and antioxidant properties of MEHSL. In vitro and in vivo assays were performed to study the effects of MEHSL on Ehrlich ascites carcinoma (EAC) cells. Chemical constituents were also analyzed by GC–MS.
Results: Total phenolic and flavonoid contents of MEHSL were found to be 26.23±0.30 and 131.13±1.40 mg/g of extract in terms of gallic acid and catechin equivalent, respectively. The MEHSL exhibited remarkable scavenging effect on DPPH, ABTS, nitric oxide scavenging activity and inhibition of lipid peroxidation with IC50 values 6.768, 11.54, 29.84 and 17.55, respectively, compared to the positive control, catechin. In vitro cytotoxic study shows that MEHSL reduced the viability of Ehrlich ascites carcinoma (EAC) cells in dose dependent manner. In in vivo, significant inhibition of EAC cells growth and proliferation, reduction of tumor weight and increased of survival time of EAC-bearing mice were noted following by intraperitoneal MEHSL treatment at the doses of 5 and 10 mg/ kg/day (p <0.05). Moreover, the MEHSL also altered the depleted hematological parameters like RBC, WBC, and percentage of hemoglobin (Hb %) of EAC bearing mice towards normal. Furthermore, administration of MEHSL induced apoptosis of EAC cells as observed in Hoechst-33342 stained cells under fluorescence microscope. Hexadecanoic acid, methyl ester (2.845%), hexadecanoic acid (9.858%), linolenic acid methyl ester (2.142%), 9,12- -octadecadien -1-Ol (2.063), alpha-glyceryl linolenate (6.138), arachidic acid (23.280%) and phthalic acid, 6-ethyloct-3-yl 2-ethylhexyl ester (74.926%) were identified as the major constituents of MEHSL by GC-MS analysis.
Conclusion: Findings of the present study suggested that the leaves of H. sabdariffa can therefore be considered as a promising resource in cancer chemotherapy.
Keywords: Hibiscus sabdariffa, Antioxidant, Anticancer, Apoptosis.
Introduction
Cancer causes largest mortality, which is probably over 6 million lives every year to be claimed in the world.1 Neoplastic transformation occurs due to contact of body with several factors such as tobacco, chemicals, radiation, pathogenic organism from environment and mutations, hormones, altered immunity generated in body.2 Chemotherapy is one of the most promising criteria to treat or control cancer.
Since chemical anticancer drugs are found to have adverse effect on body, nowadays people are motivated towards natural agents to treat cancer. Fruits and vegetables are potential source of natural anticancer agents that protect our body against cancer by scavenging and neutralizing free radicals, altering the metabolic pathway and dysregulating hormonal action involving in cancer development.3-4
Hibiscus sabdariffa L. is a lush shrub belonging to the family of Malvaceae. This Shrub is seen to have a green or reddish stem with small branches and pink, purple, or creamy white flowers, with red or white corpulent calyces that form the fruits.5 In Africa, people intake the leaves of H. sabdariffa as vegetables in the form of soups and sauces.6 The previous study showed that the leaf extract of H. sabdariffa occupied anti-hyperammonemic, anti-atherosclerotic, anti-filarial and anti-hyperlipidimic properties.7-10 The antineoplastic potential of leaves from H. sabdarifa against EAC cells is not available in the article. Therefore, the aim of the present study was to explore the antioxidant, cytotoxic and anticancer potential of methanolic extract of leaves from H. sabdariffa against EAC cells bearing mice. The study also analyzed the chemical constituents of methanol extract of H. sabdariffa leaves.
Materials and Methods
Plant materials: The leaves of H. sabdariffa (taxonomically identified with the help of department of Botany, University of Rajshahi) were collected from the district of Rajshahi, Bangladesh in February- march in 2016 during the stages of infant, mature and ripen. The voucher sample (No. 12) of this collection was deposited for further reference.
Extract preparation: The air-dried and coarsely powdered plant materials were successively macerated with methanol (250 g powder in 500 ml methanol) at room temperature. The obtained liquid methanolic extract was evaporated using a rotary evaporator at 40 0C followed by filtration to have dried methanolic extract (about 10% yield).
Chemicals and reagents: Roswell Park Memorial Institute (RPMI) 1640-medium and MTT were from Sigma-Aldrich, USA. Penicillin-streptomycin and fetal calf serum from Invitrogen (USA). Trypan blue and all other chemicals/reagents were of analytical grade obtained commercially.
Determination of total phenolic content: Total soluble phenolic remaining in MEHSL were ascertained with Folin-ciocalteau reagent using Gallic acid as a standard reference.11 A mixture of 500 µl MEHSL solution and 2.5 ml of Folin-ciocalteau reagent were placed in test tubes and vigorously homogenized. After 5 min, 2.5 ml of 7.5% (w/v) Na2CO3 were added and ultimately, allowed to be incubated at room temperature for 30 min. The absorbance was then measured at 760 nm and results were revealed in gm. of Gallic acid equivalent per gm. of dry weight.
Determination of total flavonoid content: To quantify flavonoid content using AlCl3 solution, catechin was applied as a reference standard.12 Briefly, 500 µl of MEHSL was interlarded with 5 ml of distilled water and 0.3 ml of NaNO3 solution. After 5 min of incubation at room temperature, 0.6 ml of 10% AlCl3 solution was added. Subsequently, 2 ml of 1M NaOH solution was added to the mixture and finally incubated for 15 min at room temperature. The absorbance was then measured at 510 nm and the amount of total flavonoid content was expressed in terms of mg equivalent of catechin per g of dry weight.
Determination of DPPH free radical scavenging activity Photoconstituents that remain in plant extract donate hydrogen to scavenge radicals. Hydrogen donation or free radical scavenging ability of MEHSL was measured using the stable radical DPPH and ascorbic acid as a reference standard.13 Briefly, 3 ml methanolic solution of DPPH was added to 1 ml of MEHSL solution in methanol at different concentrations and allowed to be incubated at room temperature for 30 min. The absorbance was then measured at 517 nm. Catechin was used as positive control. The percentage of free radicals, inhibited by the extract indicates the DPPH free radical scavenging activity that was calculated according to the following equation: SA% = [(Absorbance of control– Absorbance of sample) /Absorbance of control] ×100
Scavenging effect of the ABTS radical: ABTS radical cation assay was conducted through preparing a solution with equal volume of 7 mM ABTS and 2.45 mM potassium persulfate and subsequently, incubating to generate ABTS. + Cation in the dark at room temperature for 12h.14 This solution was delivered to be diluted with water to obtain an absorbance at 734 nm of 0.70±0.02. 3 ml of this prepared solution with 1ml of MEHSL solution at distinct concentrations were interlarded vigorously and finally, the absorbance was measured at 734 nm after staying for 6 min. Catechin was used as positive control. The percentage of inhibition was measured similar to that of DPPH assay.
Nitric oxide radical scavenging activity: Nitric oxide evolved from nitroprusside was measured by greiss reaction. Sodium nitropruside is decomposed to generate NO in aqueous solution at physiological pH. This NO interacts with oxygen to produce stable nitrate and nitrite ions, the amount of which can be estimated by greiss reaction.15 Briefly, 2 ml of 10 mM sodium nitropruside in phosphate buffered saline was mixed with 0.5 ml of MEHSL solution at distinct concentrations and allowed to be incubated at room temperature for 150 min. Then, 0.5 ml of greiss reagent (1 ml of o.33% sulfanilic acid reagent in 20% glacial acetic acid) was added to the test solutions and kept at room temperature for 5 min. Subsequently, 1 ml of 0.1% napthylethylenediamine dichloride was added and allowed to be incubated again for 30 min at room temperature. The absorbance was then measured at 546 nm. Catechin was considered to be the reference standard. The percentage of inhibition was measured similar to that of DPPH assay.
Determination of inhibition of lipid peroxidation: This assay system demonstrates the ability of photoconstituents to induce lipid peroxidation that was measured by a method described previously.16 Briefly, 1ml of reaction solution containing 0.5 ml liver homogenate, 100 µl of 10 mm FeSO4, 100 µl of 0.1 mM acetic acid and 0.3 ml of MEHSL at distinct concentrations was incubated at 37 0C for 20 min and the mixtures were further incubated at 100 0C for 15 min followed by the addition of 28% TCA and 1.5 ml of 1% TBA and cooled at room temperature. Catechin was used as a reference standard. Finally, the absorbance was measured spectrophotometrically at 532 nm and the percentage of inhibition was calculated by the similar equation used in DPPH assay. Catechin was used as positive control.
Test animals: Swiss albino mice of either sex, 3-4 weeks of age, weighting between 20-25 g were collected from the animal research branch of the international center for diarrheal Diseases and Research, Bangladesh (ICCDRB). They were maintained under standard laboratory conditions (temperature 22-2, humidity 55-3%) with 12h day- night cycle and also provided with standard dry pellet diet and water ad libitum. Protocol used in this study for the use of mice as a animal model for cancer research was approved by the Institutional Animal, Medical Ethics, Biosafety and Biosecurity Committee (IAMEBBC) for Experimentations on Animal, Human, Microbes and Living Natural Sources, (225/320- IAMEBBC/IBSc), Institute of Biological Sciences, University of Rajshahi, Bangladesh.
Cell culture: Ehrlich ascites carcinoma cells were collected from Institute of chemical Biology, India and maintained by weekly (i.p.) inoculation of 1×106 cells / mouse under standard laboratory conditions. For in vitro cytotoxic assay, EAC cells were cultured in Roswell Park Memorial Institute (RPMI-1640) media provided with 10% fetal calf serum, and 1% (v/v) penicillin-streptomycin in an incubator with 5% CO2 at 37°C.
MTT colorimetric assay: MTT colorimetric assay was performed to measure the cytotoxicity of MEHSL against EAC cells.17 Briefly, 2.5×105 EAC cells were plated in 200 µl RPMI media per in a 96 well plate at different concentrations of MEHSL solution and kept in CO2 incubator for 24h at 37°C. After the incubation period, aliquot was aspirated and 180 µl of phosphate buffered saline (PBS) and 20 µl of MTT were added to each well to be incubated further for 8h at 37°C. Subsequently, the aliquot was removed, and 200µl acidic isopropanol was added and again incubated at 37 0C for 1h. Finally, the absorbance was taken at 570 nm in micro-titer plate reader.
In vivo cell growth inhibition: This study was conducted on Swiss albino mice of four groups and every mouse of each group containing 6 in number was inoculated with 1.5×106 EAC cells on day 0. After 24h of tumor cells inoculation, MEHSL at doses of 5 and 10 mg/kg per day were given to every mice of group 1-2 respectively. Bleomycin at the dose of 0.3 mg/kg was given to group-3 while group-4 was used as control mice. After sacrificing the mice on day 6, total interparitoneal cells were harvested by saline. Viable tumor cells per mouse of the treated group were compared with those of the control (XDS-IR, Optica, and Bergamo, Italy).18
Determination of average tumor weight and survival time: These parameters were measured under similar experimental conditions as stated in the previous experiment.19 Briefly, four groups of Swiss albino mice (six in each group) were used. For therapeutic evaluation, 1.5×106 EAC cells per mouse were inoculated to each group of mice on day zero. Treatment was given after 24h of inoculation and continued for 10 days. Groups 1–2 received MEHSL at different concentrations; group 3 was treated with bleomycin and group 4 was used as control. The daily weight change and survival time of each mouse were recorded throughout 10 days of treatment.
Haemotological parameters: To assess the haemotological parameters, four groups of mice of which each group contain 6 in number were taken. Group one; normal mice (without any treatment), group two: EAC bearing control mice (only EAC treated), group three and four: EAC bearing mice treated with MEHSL at dose 5 and 10 mg/kg/day respectively. On the 12th day, after tumor transplantation, tail vein blood was collected which was used to measure hematological profile.20
Cell morphologic change and nuclear damage: Fluorescence microscopic observation was undertaken to ascertain cellular apoptosis in the absence and presence of MEHSL at dose of 10 mg/ kg. Briefly, the EAC cells were collected from the intraparitoneal space of both group of non-treated and treated mice with MEHSL and then, stained with Hoechst-33342 solution at 37 0C for 10 min in dark and subsequently, washed with phosphate buffered saline (PBS). Finally, fluorescence microscopic observation was carried out to determine morphological changes.17
Brine shrimp lethality bioassay: Brine shrimp lethality bioassay was conducted to explore the cytotoxicity of MEHSL against Artemia salina in a 1-day in vivo assay. Briefly, 3 mg of MEHSL was dissolved in 0.6 ml of distilled water to get a concentration of 5 µg/ml and the prepared solution was serially diluted to obtain 6.25, 12.5, 25, 50 and 100 µg/ml concentrations. After 24h incubation, the vials were observed using a magnifying glass and noted. From these data, the percentage of mortality of the nauplii was calculated for each concentration and the LC50 value was estimated using Probit analysis as described in the literature.21
GC-MS analysis of chemical constituents: Separation and identification of the constituents of methanol extract were performed by GC–MS agilent 6890 N gas chromatography hooked to agilent 5973 N mass selective detector. They equipped with a flame ionization detector and capillary column with HP-5MS (30 m × 0.25 mm × 0.25 mm).
In GC settings: the initial oven temperature was set at 60°C for 1 min and ramped at 10°C min-1 to 180°C for 1 min and then ramped at 20°C min-1 to 280°C for 15 min. The temperature of the injector was controlled at 270°C. The samples (1 ml) were injected neat, with a split ratio of 1: 10. Helium was used as the carrier gas at a flow rate of 1.0 ml min -1. Spectra were scanned from 20 to 550 m/z at 2 scans s-1. Identification of most constituents by gas chromatography was done by comparing their retention indices with those reported in the literature or with those of authentic components available in database.
Statistical analysis: The data were analysed by one- way ANOVA (analysis of variance) followed by multiple comparisons using Dunnett’s post hoc test using SPSS software of 16 version. All results were represented as mean ± standard deviation (SD). Differences at p<0.05 level were considered to be statistically significant.
Results
Total phenolic and Flavonoid content: Our study showed that the phenolic and flavonoid content in MEHSL were 26.23±0.30 mg/g gallic acid equivalent and 131.13±1.40 mg/g catechin equivalent, respectively (table I).
1MEHSL were found to exhibit the antioxidant activity with the IC50 values 6.768, 11.54, 29.84 and 17.55 µg/ml for DPPH, ABTS, nitric oxide and lipid peroxidation scavenging ability respectively while 2.651, 3.746, 3.23 and 7.52 µg/ml were for catechin (table I).
Sample |
TPC (mg of gallic acid equivalent/g of extract) |
TFC (mg of catechin equivalent/g of extract) |
DPPH radical scavenging activity (IC50 values in µg/ml) |
ABTS radical scavenging activity (IC50 values in µg/ml) |
Nitric oxide scavenging activity (IC50 values in µg/ml) |
Lipid peroxidation inhibition activity (IC50 values in µg/ml) |
|---|---|---|---|---|---|---|
MEHSL |
26.23±0.30 |
131.13±1.40 |
6.768 |
11.54 |
29.84 |
17.55 |
Catechin |
- |
- |
2.651 |
3.746 |
3.23 |
7.52 |
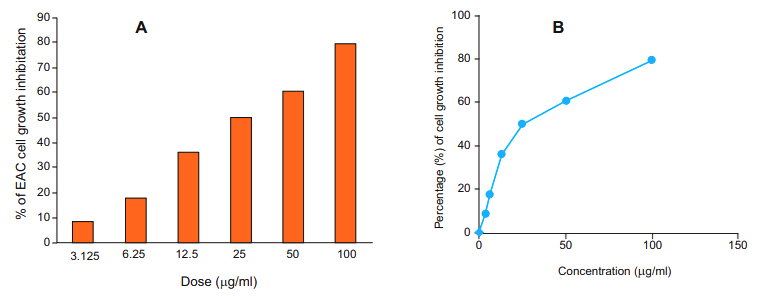
In vitro cytotoxic assay: To investigate the cytotoxic properties of MEHSL, EAC cells were incubated with extract (3.125-100 µg/ml) at 37 °C for 24 h, and cell viability was calculated by MTT assay. MEHSL induced EAC cell death in a dose dependent manner (figure 1A). (Figure 1A & 1B).
A reduced cell growth was observed with MEHSL at a concentration as low as 3.125 µg/ml which markedly increased with increasing concentration of MEHSL compared to control. A strong inhibition (60.87%) of EAC cell growth was observed at concentration 50 ìg/ml which is further increased (79.89%) at concentration 100 ìg/ml of MEHSL. The IC50 value of the MEHSL was determined as 26.15 µg/ml against EAC cell (figure. 1B).In vivo cell growth inhibition: In vivo cell growth inhibition was ascertained with MEHSL at various doses 5 and 10 mg/ kg compared to that of bleomycin at dose 0.3 mg/ kg (table II). The MEHSL at dose 10 mg/ kg per mice/day showed maximum cell growth inhibition (70.15%; p<0.05) while that of Bleomycin at dose 0.3 mg/kg per mice/day was (91.82%; p<0.05).
Group No. |
Treatment |
Viable EAC cells on day 6 after inoculation (x 107 cells/ml) |
Percentage (%) cell growth inhibition |
|---|---|---|---|
1 |
EAC + Control |
4.77±0.44 |
- |
2 |
EAC+ MEHSL (5 mg/kg) |
1.92±0.21* |
59.85±2.36 |
3 |
EAC+ MEHSL (10 mg/kg) |
1.40±0.15* |
70.15±3.14 |
4 |
EAC + Bleomycin (0.3 mg/kg) |
0.39±0.09* |
91.82±1.14 |
Average of tumor weight and survival time: Ehrlich ascites carcinoma cells cell-bearing mice were treated with methanol extract at the doses of 5 and 10 mg/ kg/day for 10 days and their tumor weight was calculated. It was observed that the extract and bleomycin reduces tumor burden approximately in a similar manner (Table III). It was also noticed that treatment of tumor-bearing mice with methanol extract at doses 5 and 10 mg/kg/day resulted in significant (p <0.05) increase of life span, which were 30.04% and 65.94%, respectively, compared with that of control mice (table III).
Group No. |
Treatment |
MST (in days) |
%ILS |
Body weight gain (g) after 15 days |
|---|---|---|---|---|
1 |
EAC + Control |
20.25±1.70 |
- |
16.00±1.41 |
2 |
EAC + MEHSL (5 mg/kg) |
26.25±0.95* |
30.04±2.68 |
5.12±0.85* |
3 |
EAC + MEHSL (10 mg/kg) |
33.50±1.29* |
65.94±4.24 |
4.37±0.57* |
4 |
EAC + Bleomycin (0.3 mg/kg) |
39.25±0.79* |
93.82±3.45 |
3.62±0.47* |
Haemotological parameters: The hematological parameters of treated and untreated mice were studied. In non-treated EAC bearing mice, these parameters showed effect consistent with physiological deterioration as compared with the control mice due to toxicity of EAC cells. However, these deteriorated parameters became reversed toward the normal ranges when MEHSL supplementation was given to them at the dose used in this study (table IV).
Name of Exp. |
RBC Cells (x109) /ml |
WBC Cells (x106) |
/ml |
% of Hb gm/dl |
|---|---|---|---|---|
Normal mice |
6.67 ± 0.23 |
8.75 ± 0.53 |
14.48 ± 0.52 |
|
EAC+Control |
2.00±0.18 |
42.00±5.71 |
9.50±0.73 |
|
EAC+MEHSL (5 mg/kg) |
2.90±0.17* |
11.75±2.06* |
13.1±0.95* |
|
EAC+MEHSL (10 mg/kg) |
4.68±0.50* |
7.75±1.70* |
15.35±0.66* |
|
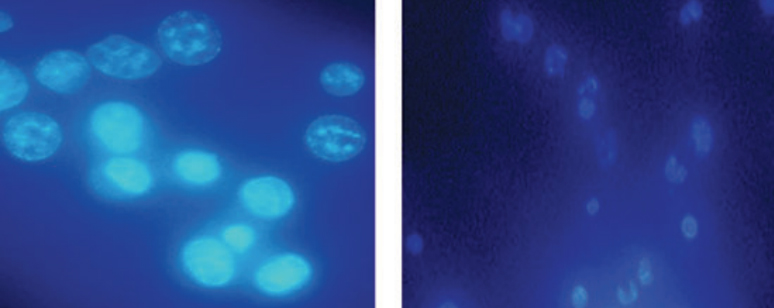
Morphological change and Nuclear Damage: Fluorescence microscopic observation revealed that EAC cell nuclei were round, regular and homogenously stained with Hoechst 33342 in solvent treated group while EAC cells treated with MEHSL at dose 10 mg/ kg for 5 days showed nuclear condensation, fragmentation and membrane blabbing compared to the normal mice as shown in Figure 2A and 2B respectively. It indicates that the MEHSL could induce apoptosis of EAC cells. (Figure 2).
Brine shrimp lethality bioassay (LC50): The brine shrimp lethality bioassay was carried out to assess the in vitro cytotoxic effect of MEHSL. Percent of mortality of nauplii were increased with the increasing concentration of MEHSL and the medium lethal concentration (LC50) was found to be 21.32 µg/ml.
Peak# |
Name of compound |
Retention Time |
Percentage composition |
|---|---|---|---|
1 |
Hexadecanoic acid, methyl ester |
20.327 |
2.845 |
2 |
Hexadecanoic acid |
20.826 |
9.858 |
3 |
Linolenic acid methyl ester |
22.586 |
2.142 |
4 |
9,12- -octadecadien -1-Ol |
22.992 |
2.063 |
5 |
alpha-glyceryl linolenate |
23.084 |
6.138 |
6 |
Arachidic acid |
23.280 |
2.029 |
7 |
Phthalic acid, 6-ethyloct-3-yl 2-ethylhexyl ester |
28.181 |
74.926 |
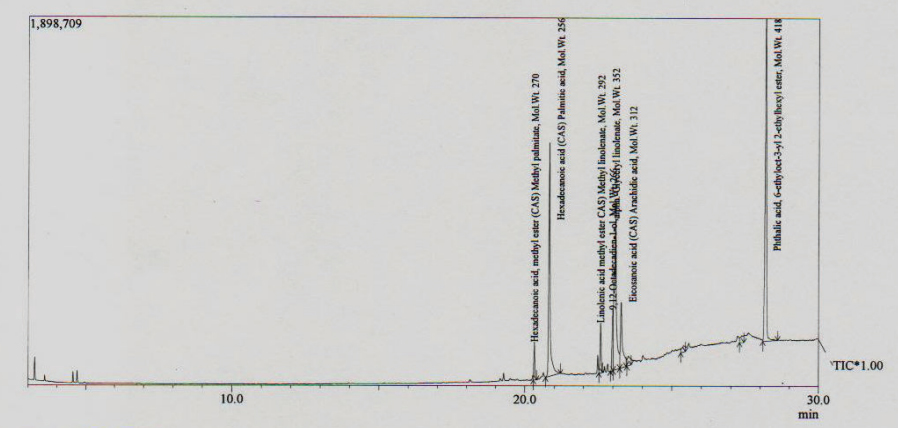
Chemical constituent of MEHSL: A total of seven components as hexadecanoic acid, methyl ester (2.845%), hexadecanoic acid (9.858%), linolenic acid methyl ester (2.142%), 9,12- -octadecadien -1-Ol (2.063), alpha-glyceryl linolenate (6.138), arachidic acid (23.280%) and phthalic acid, 6-ethyloct-3-yl 2- ethylhexyl ester (74.926%) were identified in the methanol extract, accounting for 100% of the extract (table IV & Figure 3).
Discussion
In the present study, we demonstrated that MEHSL contains a significant amount of phytochemicals (total phenolics and flavonoids) and possesses antioxidant and a strong free radical scavenging activity (table 1). In previous reports, it has been supported that medicinal plants that are affluent with phenols and polyphenolic compounds can play a crucial role in corresponding reactive oxygen species and acts as antioxidant.22 Many natural compounds of plant- derived extracts have crucial roles in corresponding the intracellular redox status and antioxidant function. Polyphenols and flavonoids which are the most plentiful component in plant kingdom, have anticancer property.23 Therefore, natural compounds with antioxidants upshots are imperative therapeutic forecast for cancer.24 In this study, we scrutinized the antioxidant prospective of MEHSL through diverse in vitro models for the first time. These evidences help us to state that the antineoplastic properties of MEHSL may be due to the presence of phytoconstituents with antioxidative activity.
Cancer involves uncontrolled proliferation of tumor cells. Our present study was conducted on EAC bearing mice to explore the anticancer activities of MEHSL. Given that Ehrlich Ascites Carcinoma (EAC) rapidly grows with very aggressive behavior.25 MTT assay was performed in the present study where MEHSL was found to inhibit EAC cells proliferation in a dose-dependent manner (figure 1A). Numerous studies performed MTT assay previously to evaluate the in vitro capacity of an extract or a compound to inhibit the growth of cancer cell and the results obtained from those studies support the findings of the present study indicating the probable use of the present extract as an anticancer agent.17
Tumor cell growth inhibition, enhancement of lifespan of tumor bearing mice, reduction of tumor weight, as well as hematological parameters are considered to be very important and promising tool to arbiter a potent anticancer drug.26 In this study, untreated EAC cell- bearing mice gained body weight rapidly due to tumor burden but the treatment of the EAC-cell bearing mice with MEHSL reduced body weight gain significantly, inhibited cell growth sufficiently and increased life span remarkably (table II).
The MEHSL also reduced the tumor burden of EAC- bearing mice remarkably, as MEHSL-treated mice showed only 72.68% augmentation in body weight compared to that of only EAC-inoculated mice, whereas that of bleomycin-treated mice increased by 77.37%. Moreover, MEHSL supplementation increased the life span of tumor-bearing mice very effectively, compared to that of control and bleomycin–treated EAC-bearing mice (table III) and similar results were also observed in EAC-bearing mice treated with plant extract.19
In cancer chemotherapy, the major problems are myelosuppression and anemia. In anemia, percentage of RBC or hemoglobin in tumor bearing mice is reduced probably either due to iron deficiency or due to hemolytic or myelopathic conditions.27 Administration of the methanol extract in EAC cell- bearing mice reversed back all the altered hematological parameters more or less to normal level (table IV) and this result is similar with that of previous studies19 where EAC cell-bearing mice were treated with bioactive compounds suggesting the protective action of MEHSR on the hemopoetic system.
Apoptosis, an intrinsic cell-suicidal mechanism regulated by various cellular signaling pathways, is characterized by cell shrinkage, condensation of chromatin and apoptotic body formation.17 In this study, EAC cells treated with MEHSL showed characteristics features of apoptosis (cell membrane blebbing, nuclear condensation and fragmentation.) On the Other hand, regular and round shape was found in control cell (figure 2). Apoptosis of EAC cells was also reported by using different plant extracts.17
Hexadecanoic acid, methyl ester (2.845%), hexadecanoic acid (9.858%), linolenic acid methyl ester (2.142%), 9,12- -octadecadien -1-Ol (2.063), alpha- glyceryl linolenate (6.138), arachidic acid (23.280%) and phthalic acid, 6-ethyloct-3-yl 2-ethylhexyl ester (74.926%) were identified as the major components of MEHSL by GC-MS analysis (table V). Hexadecanoic acid was found to have potential antioxidant activity.28 The antitumor properties of the extracts may be caused due to presence of these compounds.
Conclusion
The present study concluded that MEHSL was effective in inhibiting the growth of EAC in vitro and vivo conditions. The possible mechanism of this antineoplastic effect may be due to the rich polyphenolic and flavonoid content of MEHSL and its antioxidant properties. It may be possible that as natural antioxidants polyphenolic and flavonoid type compounds reinforce the endogenous antioxidant defense from reactive oxygen species (ROS) ravage and restore the optimal balance by neutralizing the reactive species. However, our future studies will be carried out to identify the compounds involved in antioxidant and antineoplastic activities.
Acknowledgments
The authors thank IICB, Kolkata, India, authority for providing the EAC cells and the International Centre for Diarrhoeal Disease Research, Bangladesh for supplying the Swiss albino mice.
References
- Abdullaev FI, Luna RR, Roitenburd BV, Espinosa AJ. Pattern of childhood cancer mortality in Mexico. Arch Med Res. 2000; 31:526-531.
- Tanaka T. Chemoprevention of human cancer: biology and therapy. Crit Rev Oncol Hematol. 1997; 25:139-74.
- Hung HC, Joshipura KJ, Jiang R, Hu HU, Hunter D, Smith- Warner SA, Colditz GA, Rosner B, Spiegelman D, Willett WC. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004; 96:1577-584.
- Craig WJ. 1999. A vegetarian Way to Better Health Golden Harvest Books, Nutrition and wellness. Berrian Springs.
- Sarkar MR, Hossen, SM, Howlader MSI, Rahman MA, Dey A. Anti-diarrheal, Analgesic and Anti-microbial activities of the plant Lal mesta (Hibiscus sabdariffa): A review. Int J Pharm Life Sci. 2012; 1: 2.
- Chen J, Wang C, Wang C, Sheu J, Lin C, Lin H. Hibiscus sabdariffa leaf polyphenolic extract inhibits LDL oxidation and foam cell formation involving up-regulation of LXRá/ ABCA1 pathway. Food Chem. 2013; 14:397-406.
- Essa MM, Subramanian P. Hibiscus sabdariffa affects ammonium chloride induced hyperammonemic rats. Evid Based complement Altern Med. 2007; 4:321-25.
- Ochani PC, D’Mello P. Antioxidant and Antihyperlipidemic activity of Hibiscus sabdariffa Linn. Leaves and calyces extract in rats. Indian J Exp Biol. 2009; 47:276-82.
- Saxena K, Dube V, Kushwaha V, Gupta V, Lakshmi M, Mishra S, Gupta S, Arora A,, Lakshmi V, Sharma RK, Jain GK, Murthy PK. Antifilarial efficacy of Hibiscus sabdariffa on lymphatic filarial parasite Brugia malayi. Med Chem Res. 2011; 20:1594–1602.
- Gosain S, Ircchiaya R, Sharma PC, Thareja S, Kalra A, Deep A, Bhardwaj TR. Hypolipidemic effect of ethanolic extract from leaves of Hibiscus sabdariffa L. in hyperlipidemic rats. Acta Pol Pharm. 2010; 67:179-84.
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am J Enol Viticult. 1965; 16:144-58.
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002; 50: 3010-3014.
- Choi HY, Jhun EJ. Lim BO. Application of flow injection- chemilumineacence to the study of radical scavenging activity in plants. Phytother Res. 2000; 14:250-53.
- Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004; 74:2157-84.
- Sivakumar P, Sambath KR, Sivakumar T, Perumal P, Jayakar B. In vitro antioxidant activity of ethanol extract of Triumfetta rhomboidea. Int J Pharma Tech. 2010; 2:665-673.
- Liu F, Ng TB. Antioxidative and free radical scavenging activities of selected medicinal herbs. J Life Sci. 2000; 66:725-35.
- Kabir SR, Rahman MM. Amin R, Karim MR, Mahmud ZH, Hossain MT. Solanum tuberosum lectin inhibits Ehrlich ascites carcinoma cells growth by inducing apoptosis and G2/M cell cycle arrest. Tumor Biol. 2016; 37:8437-44.
- Osman MA, Rashid MM, Aziz MA, Habib MR, Karim MR. Inhibitions of Ehrlich ascites carcinoma by Manilkara zapota L. stem bark in Swiss albino mice. Asian Pac J Trop Biomed. 2011; 1:448-51.
- Habib MR, Aziz AA, Karim MR. Inhibition of Ehrlich’s ascites carcinoma by ethyl acetate extract from the flower of Calotropis gigantea L. in mice. J Appl Biomed. 2010; 8:47–54.
- Mukherjee KL. 1988. Medical Laboratory Technology. Tata Mcgraw Hill Publishing Company Limited. New Delhi. p.288-307.
- Kabir SR, Zubair MA, Nurujjaman M, Haque MA, Hasan I, Islam MF, Hossain MT, Hossain, MA, Rakib MA, Alam MT, Shaha RK, Hossain MT, Kimura Y, Absar N. Purification and characterization of a Ca(2+)-dependent novel lectin from Nymphaea nouchali tuber with antiproliferative activities. Biosci Rep. 2011; 31:465-75.
- Formica JV, Regelson W. Review of the biology of quercetin and related bioflavonoids, Food Chem Toxicol. 1995; 33:1061-1080.
- Fotsis T, Pepper MS, Aktas E, Breit S, Rasku S, Adlercreutz H. Flavonoid, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997; 57:2916-21.
- Lamoral-Theys D, Pottier L, Dufrasne F, Neve J, Dubois J, Kornienko A, Kiss R, Ingrassia L. Natural polyphenols that display anticancer properties through inhibition of kinase activity. Curr Med Chem. 2010; 17:812-25.
- Segura JA, Barbero LG, Marquez J. Ehrlich ascites tumour unbalances splenic cell population and reduces responsiveness of T cells to Staphylococcus aureus entero toxin B stimulation. Immunol Lett. 2000; 74:111-15.
- Hirsch J. An anniversary for cancer chemotherapy. JAMA. 2006; 296:1518–1520.
- Hogland HC. Heamatological complications of cancer chemotherapy. Semi Oncol. 1982; 9:95-102.
- Rajalakshmi K, Mohan VR. GC–MS analysis of bioactive components of Myxopyrum serratulum A.W. Hill (Oleaceae). Int J Pharm Sci Rev Res. 2016; 38:30-35.
Department of Biochemistry and Molecular Biology, University of Rajshahi, Rajshahi-6205, Bangladesh.
yeasmin_bio@yahoo.com
 0000-0001-8751-9515
0000-0001-8751-9515
Submission
24 June 2020
Accepted
30 July 2021
Published
01 August 2021
Apply citation style format of Bangladesh Medical Research Council
Issue
Vol 47 No 2 (2021)
Section
Research Articles
Ethical Clearance
Institute of Biological Sciences, University of Rajshahi, Bangladesh.
Financial Support
None
Conflict of Interest
We declare that we have no conflict of interest.