Zulaikha Mohamed
Department of Obstetrics and Gynaecology, Indira Gandhi Memorial Hosopital, Male, Maldives.
Shirin Akter Begum
Department of Gynaecological Oncology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh.
Tasfia Mahmud
Department of Pharmacology, Sirajul Islam Medical College, Dhaka, Bangladesh.
Mehriban Amatullah
Department of Gynaecological Oncology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh.
Afroza Khanom
Department of Gynaecological Oncology, National Institute of Cancer Research Institute and Hospital, Dhaka, Bangladesh.
Keywords: CA-125, Surgical staging, Ovarian cancer, Serous tumour
DOI: 10.3329/bmrcb.v47i2.57768
Abstract
Background: Ovarian cancer is one of the leading cause of mortality and morbidity of gynaecological malignancies. Lack of early demonstration of symptoms and lack of effective screening tests, ovarian tumours are usually diagnosed at an advanced stage. Different studies shows the association of pre-operative serum CA125 level with the surgical staging of ovarian cancer.
Objective: To find out the correlation of pre-operative level of serum CA 125 with the surgical staging (FIGO-2014) of ovarian cancer.
Methodology: This cross sectional study was carried out among 81 subjects in the department of gynaecological oncology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, from August 2018 to September 2019. Sampling technique was purposive. All consecutive patients admitted at department of gynaecological oncology, BSMMU with diagnosed ovarian cancer were included in this study. Data were collected and documented on a preformed and pretested structured questionnaire. Clinical examinations and relevant investigations were done and recorded accordingly.
Result: Mean age of the study subjects was 45.7 ± 15.2 years with a range of 13-75 years. Mean BMI was 23.3 ± 3.0 kg/m2 and mean age of marriage was 16.1 ± 2.8 years. Maximum study subjects had stage III (44.4%) followed by stage II (23.5%), stage I (21.0%) and stage IV (11.1%). Preoperative CA-125 was >35 U/ml in 90.1% cases. Serum CA-125 was elevated at the advanced stage of ovarian cancer. Maximum study subjects had serous tumors (70.4%) followed by mucinous tumors (11.5%), endometrioid adenocarcinoma (7.4%), malignant teratoma (4.9%) and clear cell tumors (3.7%). Serum CA-125 had significant positive correlation with surgical stages of ovarian epithelial cancer.
Conclusion: Surgical stage of epithelial ovarian cancer significantly correlates with CA-125.
Keywords: CA-125, Surgical staging, Ovarian cancer, Serous tumour.
Introduction
Ovarian cancer is an alarming health problem in Bangladesh. The annual mortality rate per 100,000 people from ovarian cancer in Bangladesh has raised by 40.3% since 1990, an average of 1.8% a year. Globcan predicts a change in the reported incidence of ovarian cancer from 2912 in year 2012 to 3132 in 2015. Recurrent high-grade ovarian cancer is usually associated with short term survival.1 Disease stage at diagnosis is a strong prognostic variable for predicting patient outcome in ovarian cancer. Patients with International Federation of Gynecology and Obstetrics (FIGO) stage III ovarian cancer, indicating tumor dissemination and seeding of the peritoneal lining outside of the pelvis, have a 5-year survival rate of approximately 35%. This survival rate decrease to less than 10% in patients diagnosed with stage IV ovarian cancer, where disease has spread to distant metastasis.2 Therefore, early detection and appropriate management prevent the fatal outcome of ovarian cancer.
Ovarian carcinomas relate to highest death rate in gynaecological malignancies as absence of symptoms shield the disease in the early stage. Biomarkers are the important tools that are capable of predicting progression, risk stratification and overall therapeutic benefit to fight against this deadly disease.3 The present status of biomarkers used in the context of ovarian cancer is addressed. Importance is given to new interpretations of the aetiology of ovarian cancer.4
CA125 is a glycoprotein first described in 1981. It is a tumour marker of ovarian malignancy and it is derived from both coelomic and mullerian epithilial. It is approved by US FDA. It has two major antigen domain components, they are OC125 and M11. CA125 is a biomarker that has potential utility across the spectrum: for risk assessment, early detection, diagnosis, prognosis, monitoring and therapy. Overall sensitivity of serum CA125 level to cyto-histological expression is 100% and the specificity is 86% with positive and negative predictive value of 74% and 100% respectively.5 CA125 level 35 U/mL is considered as the cut-off value when evaluating serum CA125 ovarian cancer.6An immature teratoma is a very rare tumor, representing 1% of all teratomas, 1% of all ovarian cancers, and 35.6% of malignant ovarian germ cell tumors.7
Whenever malignancy is suspected, a staging laparotomy should be carried out. The importance of thorough surgical staging cannot be overemphasised because subsequent treatment will be determined by the stage of the disease. Therefore, we have investigated the histological characteristics and stage of ovarian cancer and have correlated with preoperative level of serum CA125. We also tried to find out role of CA125 in identifying different stages of ovarian cancer.
Ovarian cancer ranks 7th in both incidence and mortality among women worldwide. In 2012, an estimated 238,000 women were diagnosed with and 151,000 women died from ovarian cancer, representing 4% of all new cancer diagnoses and 4% of cancer deaths among women.8 Since the early part of the 20th century, it has been recognized that ovarian cancer is not a single disease, but comprised of various histologically different tumour types. Ovarian cancers have generally been divided into epithelial and non-epithelial groups for many years. Epithelial, germ cell and sex cord-stromal tumours are the commonest types of ovarian cancer. They can be further subdivided into distinct histological subtypes. The developmental pathway and clinical prognosis for a particular ovarian tumour depends upon the histological subtype.9 Type I epithelial tumours include low-grade serous, endometrioid, clear cell, mucinous, squamous and transitional cell (Brenner) carcinomas. They often present at an early stage, may arise from borderline ovarian tumours or endometriosis, and typically have a good prognosis.10 Type II epithelial tumours comprise high-grade serous carcinoma, undifferentiated carcinomas and malignant mixed mesodermal tumours (carcinosarcoma). They account for around 75% of epithelial ovarian cancers, typically present at an advanced stage and have a poor prognosis.11
CA-125 (cancer antigen 125, carcinoma antigen 125, or carbohydrate antigen 125) also known as mucin 16. MUC16 is a protein that in humans is encoded by the MUC16gene.12 MUC16 is a member of the mucin family glycoproteins. CA-125 has found application as a tumor marker or biomarker that may be elevated in the blood of some patients with specific types of cancers or other conditions that are benign.13 A unique property of MUC16 is its large size. MUC16 is more than twice as long as MUC1 and MUC4 and contains about 22,000 amino acids, making it the largest membrane-associated mucin.14 MUC16 is composed of three different domains.15, An N-terminal domain, A tandem repeat domain, A C-terminal domain.
MUC16 is a component of the ocular surface, the respiratory tract and the female reproductive tract epithelia. Since MUC16 is highly glycosylated it creates a hydrophilic environment that acts as a lubricating barrier against foreign particles and infectious agents on the apical membrane of epithelial cells. Also, the cytoplasmic tail of MUC16 has been shown to interact with cytoskeleton by binding members of the ERM protein family.16 The expression of mucin 16 has been shown to be altered in dry eye, cystic fibrosis, and several types of cancers.17
CA-125 is the most frequently used biomarker for ovarian cancer detection. Around 90% of women with advanced ovarian cancer have elevated levels of CA-125 in their blood serum, making CA-125 a useful tool for detecting ovarian cancer after the onset of symptoms.18 Monitoring CA-125 blood serum levels is also useful for determining how ovarian cancer is responding to treatment (with the duration of disease- free survival correlating with the rate of fall of CA-125) and for predicting a patient’s prognosis after treatment.18,19 This is because the persistence of high levels of CA-125 during therapy is associated with poor survival rates in patients.19 Also, an increase in CA-125 levels within individuals in a remission is a strong predictor of the recurrence of ovarian cancer.20 Indeed, a rising CA-125 level may precede clinical evidence of disease relapse by an interval of 3 to 6 months.
Prognosis relates to both the initial and post-treatment CA-125 values. A preoperative value >65 U/mL suggests a poor prognosis. Persistent elevations following chemotherapy indicate a poor prognosis. The half-life of CA-125 after chemotherapy correlates with prognosis (patients with CA-125 half-life <20 days show improved survival). Time-to-normalization (rate of fall of CA-125) affects prognosis with more rapid normalization within 3 cycles of chemotherapy correlating with improved survival.21
The potential role of CA-125 for the early detection of ovarian cancer is controversial and has not yet been adopted for widespread screening efforts in asymptomatic women.22 The major issues with using the CA-125 biomarker are its lack of sensitivity, particularly for detecting early stages of ovarian cancer, and its lack of specificity, especially in premenopausal women.23
Materials and Methods
This cross-sectional study was carried out 81 cases with ovarian cancer admitted in the department of gynaecological oncology, BSSMU, from August 2018 to September 2019 among. All consecutive patients admitted with diagnosed ovarian cancer were included in this study. Women with clinically diagnosed ovarian cancer, imaging finding suggestive of ovarian cancer and patients who had image guided FNAC proven malignant ovarian cancer were included in the study. Patients with uterine leiomyoma and ectopic pregnancy, which mimics adnexal masses, patients with endometriosis, tumor with uterine fibroid, or pregnancy or other types of malignancies and patients not fit for surgical intervention were excluded from the study. Furthermore, patients who had not willingness to participate in the study.
Data collection: Data were collected by using a preformed data sheet and informed consent was taken from all cases who fulfill the inclusion criteria. Initial evaluation of the study population by history and clinical examination was performed and recorded accordingly.
Blood sample collection: Patients’ blood sample was drawn from the antecubital vein. Five milliliters blood was drawn with proper aseptic precautions. The blood sample was transferred into a clean, dry test tube and taken to the laboratory. Blood sample was centrifuged for 10 minutes at a rate of 4000rmp
Lab method (Measurement of serum CA125 concentration): The ARCHITECT CA125 II assay is a two-step immunoassay for the quantitative determination of OC125 defined antigen in human serum or plasma using CMIA technology, with flexible assay protocols, referred to as Chemiflex. Sample and OC125 coated paramagnetic microparticles were combined. The OC125 defined antigen present in the sample binds to the OC125coated micro particles. After washing M11 acridinium-labelled conjugate was added to create a reaction mixture. Following another wash cycle Pre- Trigger and Trigger solutions were added to the reaction mixture. The resulting chemiluminesent reaction was measured as relative light units (RLUs). A direct relationship exists between the amount of OC125 defined antigen in the sample and the RLUs detected by the ARCHITECT i System optics.
Statistical analysis: Statistical analysis were carried out using the Statistical Package for Social Sciences version 22.0 for Windows (SPSS Inc., Chicago, Illinois, USA). A descriptive analysis was performed for all data. The mean values were calculated for continuous variables. The quantitative observations were indicated by frequencies and percentages. ANOVA test was performed to compare CA25 level rise along surgical stage of ovarian cancer. Correlation analysis was performed using Spearsman’s rank correlation coefficient test. p value of less than 0.05 was considered significant.
Results
This was a cross sectional study which was carried out in inpatient department of gynaecological oncology to find out the correlation of pre-operative level of serum CA 125 with the surgical stage (FIGO-2014) of ovarian cancer (Figure 1).
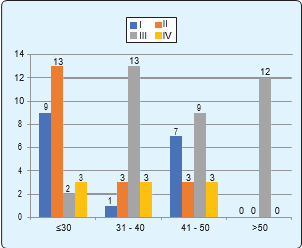
Regarding the age distribution of the study subjects, maximum (33.3%) study subjects were ≤30 years old, followed by age group 41–50 years (27.2%), 31–40years (24.7%) and >50 years (14.8%). Mean age of the study subjects was 45.7 ± 15.2 years with a range of 13-75 years (Figure 2). Majority of stage II ovarian cancer was found maximum in £30 year’s age group. Stage III was found maximum in age 31-40 years and >50 years age group (table I).
| Age (years) | Frequency (n) | Percentage (%) |
|---|---|---|
| ≤30 27 | 33.3 | |
| 31 - 40 | 20 | 24.7 |
| 41 - 50 | 22 | 27.2 |
| >50 12 | 14.8 | |
| Mean±SD | 45.7 ± 15.2 (13 - 75) | |
| n=number of study population. | ||
| Data was expressed as frequency and percentage and mean ± SD. | ||
| SD=Standard deviation. | ||
| Education | Frequency (n) | Percentage (%) |
| Primary | 41 | 50.6 |
| Secondary | 21 | 25.6 |
| Higher Secondary | 15 | 18.5 |
| Graduate | 4 | 4.9 |
| Occupation | Frequency (n) | Percentage (%) |
| Housewife | 55 | 67.9 |
| Teacher | 13 | 16.0 |
| Service holder | 6 | 7.4 |
| Student | 4 | 4.9 |
| Tailor | 3 | 3.7 |
| Monthly family income (Tk.) | Frequency (n) | Percentage (%) |
| <10,000 | 30 | 37.0 |
| 10,000 – 20,000 | 35 | 43.2 |
| >20,000 | 16 | 19.8 |
In this study, regarding evaluation of surgical stages of ovarian cancer, maximum subjects had stage III (44.4%) followed by stage II (23.5%), stage I (21.0%) and stage IV (11.1%) (Figure 3). Histopathological finding showed maximum study subjects had serous tumors (70.4%) followed by mucinous tumors (11.1%), endometrioid adenocarcinoma (7.4%), clear cell tumors (3.7%), malignant teratoma (4.9%), Brenner (1.2%) and Dysgerminoma (1.2%) (table II).
| Surgical stages of ovarian cancer | Frequency (n) | Percentage (%) | |
|---|---|---|---|
| I | 17 | 21.0 | |
| II | 19 | 23.5 | |
| III | 36 | 44.4 | |
| IV | 9 | 11.1 | |
| Type of tumors | Frequency (n) | Percentage (%) | |
| Epithelial cancer | |||
| Mucinous tumors | 9 | 11.1 | |
| Serous tumors | 57 | 70.4 | |
| Clear cell tumors | 3 | 3.7 | |
| Endometrioid adenocarcinoma | 6 | 7.4 | |
| Brenner | 1 | 1.2 | |
| Germ cell | |||
| Dysgerminoma | 1 | 1.2 | |
| Malignant teratoma | 4 | 4.9 | |
| Type of tumors | Age (years)Mean ±SD | ||
| Epithelial cancer | |||
| Mucinous tumors | 51.56 ± 21.87 | ||
| Serous tumors | 47.74 ± 11.10 | ||
| Clear cell tumors | 16.67 ± 2.52 | ||
| Endometrioid adenocarcinoma | 57.50 ± 8.21 | ||
| Brenner | 35.00 ± 0.00 | ||
| Germ cell | |||
| Dysgerminoma | 30.00 ± 0.00 | ||
| Malignant teratoma | 17.25 ± 3.10 | ||
Regarding histopathological type according to grading of ovarian tumour (Figure 4). All stage IV tumors were serous. All clear cell and Brenner tumors were stage I. All endometrioid adenocarcinoma tumors were stage III.
| Histopathological type of tumour | Stage I | Stage II | Stage III | Stage IV |
|---|---|---|---|---|
| Epithelial cancer | ||||
| Mucinous | 3 (17.6) | 3 (15.8) | 3 (8.3) | 0 (0.0) |
| Serous | 7 (41.2) | 16 (84.2) | 25 (69.4) | 9 (100.0) |
| Clear cell | 3 (17.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Endometrioid adenocarcinoma | 0 (0.0) | 0 (0.0) | 6 (16.6) | 0 (0.0) |
| Brenner | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Germ cell | ||||
| Dysgerminoma | 0 (0.0) | 0 (0.0) | 1 (2.8) | 0 (0.0) |
| Malignant teratoma | 3 (17.6) | 0 (0.0) | 1 (2.8) | 0 (0.0) |
Preoperative serum CA-125 level at different surgical stages of ovarian cancer among the study subjects showed serum CA-125 was elevated at the advanced stage of ovarian cancer. In stage IV the serum CA-125 level was 1484.00±101.34.
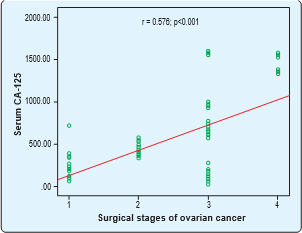
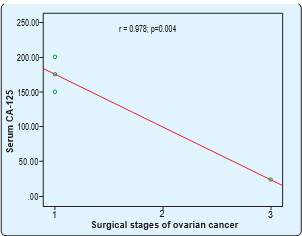
Serum CA-125 had significant positive correlation with surgical stages of ovarian cancer. Spearman’s correlation was done. Here, r= 0.576 and p=<0.001. Serum CA-125 had significant negative correlation with surgical stages of ovarian germ cell cancer. Spearman’s correlation was done. Here, r= -0.978 and p=0.004.
| Surgical stages of ovarian cancer | Serum CA-125 (mean±SD) |
|---|---|
| I | 247.18 ± 149.28 |
| II | 449.26 ± 79.33 |
| III | 505.97 ± 481.40 |
| IV | 1484.00 ± 101.34 |
| p-value | <0.001 |
Discussion
CA 125 antigen is a cell membrane glycoprotein expressed by various types of epithelial cells and it is present in patients with a variety of cancers namely breast, endometrium, gastrointestinal tract, and lung in addition to the ovarian cancer (OC) as well as in benign diseases of the uterus, liver, and gastrointestinal tract and benign tumors of the ovary and uterus.24
In present study, maximum (33.3%) study subjects were ≤30 years old followed by age group 41 – 50 years (27.2%), 31 – 40 years (24.7%) and >50 years (14.8%). Mean age of the study subjects was 45.7±15.2 years with a range of 13-75 years. Mean age of the ovarian cancer patients was almost similar in the study, they found mean age 47.5 ± 10.2 year, found mean age of the ovarian clear cell carcinoma patients was 50.8 ± 10.7 years with a range of 23-85 years, had found the mean age of his study subjects were 50.24 years which is similar to the present study.6,25
This study showed almost half of this study populations having primary education and maximum study subjects were housewife (67.9%). Majority of the study population (43.2%) had monthly income Tk. 10,000 – 20,000, only 19.8% had monthly income >Tk. 20,000. Mean BMI was 23.3 ± 3.0 kg/m2 and mean age of marriage was 16.1 ± 2.8 years, had found from his study that risk of epithelial cancer in obese woman is 30% higher than women with normal BMI.
Maximum study subjects had stage III (44.4%) followed by stage II (23.5%), stage I (21.0%) and stage IV (11.1%). Previous study done by Nayak et al (2015) showed similar results like (46.2%) had stage III disease.6 In the study of Bai et al. stage I was 45.0%, stage II was 11.5%, stage III was 35.2% and stage IV was 5.3%,25 also found maximum stage III patients (61.0%) followed by stage I (21.0%), stage II (12.0%) and stage IV (6.0%).
In this study, preoperative CA-125 was >35 U/ml in 73 (90.1%) cases. Preoperative CA-125 level was elevated (>35 U/ml) in majority (78.9%) of patients. CA-125 was >35 U/ml in 77.8% cases in the study.26 Serum CA-125 was elevated at the advanced stage of ovarian cancer. Similar finding also observed in the study.27
The present study showed histopathological distribution of the ovarian cancers, like serous tumors (70.4%), mucinous tumors (11.5%), endometrioid adenocarcinoma (7.4%), malignant teratoma (4.9%) and clear cell tumors (3.7%), found serous tumors (65.0%), clear cell tumors (12.0%), Endometroid
tumors (13.0%), mucinous tumors (5.0%), undifferentiated carcinoma (2.0% and mixed malignant mullerian tumors (3.0%). In this study, serous was 79.0%, Adenocarcinoma was 50.0%, carcinoma was 4.0%, endometrioid was 2.0% and mucinous was 1.0%, observed that serous was 27.8%, Mucinous was 48.9%, Clear cell was 15.6% and endometrioid was 5.6%.26 Most common tumor was serous adenocarcinoma (42.0%) and Mucinous adenocarcinoma (23.0%) in the study.6
In this study, serum CA-125 had significant positive correlation with surgical stages of ovarian cancer in epithelial cancer patients. But in case of germ cell ovarian cancer it showed a negative correlation. But unlike my study did not find any correlation of CA-125 with stages of ovarian cancer.6 This could be because he did the correlation for all histopathological tumour together. Preoperative serum tumor marker CA-125 levels are a useful indication of the disease.
Conclusion
It may be concluded that the preoperative serum CA125 level significantly correlates with surgical stage of ovarian cancer in case of epithelial origin ovarian cancers. Thus high level of preoperative serum CA125 level can be used as a prediction factor of ovarian cancer in case of epithelial ovarian cancer. Multicenter study should be done with large sample size for a longer duration. Other cofactors which might have an influence on stage of stage of ovarian cancer should be evaluated.
References
- Hoque ME, Karim S, Siddiqui MMR, Ahmed T. Report on Three Cases of Advance Ovarian Cancer Upon Bangladeshi Population: Successful Management with Bevacizumab Based Chemotherapy. Anwer Khan Modern Medical College Journal. 2017;8:157-61.
Doi:10.3329/akmmcj.v8i2.33675 - Coticchia CM, Yang J, Moses MA. Ovarian cancer biomarkers: current options and future promise. Journal of the National Comprehensive Cancer Network. 2008;6: 795-802.
Doi:10.6004/jnccn.2008.0059 - Muinao T, Boruah HPD, Pal M. Diagnostic and Prognostic Biomarkers in ovarian cancer and the potential roles of cancer stem cells–An updated review. Experimental cell research. 2018;362:1-10.
Doi:10.1016/j.yexcr.2017.10.018 - Ueland F. A perspective on ovarian cancer biomarkers: past, present and yet-to-come. Diagnostics. 2017;7: 1-8.
Doi:10.3390/diagnostics7010014 - Das C, Mukhopadhyay M, Ghosh T, Saha AK, Sengupta M. Correlation of cytohistlogical expression and serum level of ca125 in ovarian neoplasm. Journal of Clinical and Diagnostic Research: JCDR. 2014; 8: 41-43.
Doi:10.7860/JCDR/2014/6689.4101 - Nayak A, Shivananjaiah C, Padma K, Swarup A, Cherukumudi A, Chandran P. Corelation between the CA 125 and Staging and Histopathological Type of Ovarian Cancer. JMSCR. 2015;3: 8269-74.
Doi:10.18535/jmscr/v3i11.26 - Alwazzan AB, Popowich S, Dean E, Robinson C, Lotocki R, Altman AD. ”Pure Immature Teratoma of the Ovary inAdults”. International Journal of Gynecological Cancer. 2015:25: 1616–22.
Doi:10.1097/IGC.0000000000000541 - Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer. 2013 [cited 18 August 2018].
- Kurman RJ, Carcangiu ML, Herrington S, Young RH. WHO classification of tumours of female reproductive organs. IARC. 2014.
- Kurman, R.J. and Shih, I.M., 2016. The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. The American journal of pathology. 186:733-47.
Doi:10.1016/j.ajpath.2015.11.011 - Kurman RJ, Shih IM. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer-shifting the paradigm. Human Pathology. 2011;42: 918-31.
Doi:10.1016/j.humpath.2011.03.003 - Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen identification as a new mucin, MUC16. Journal of Biological Chemistry. 2001; 276: 27371-75.
Doi:10.1074/jbc.M103554200 - BastJr RC, Xu FJ, Yu YH, Barnhill S, Zhang Z, Mills GB. CA 125: the past and the future. The International journal of biological markers. 1998;13: 179-87.
PMID:10228898 - Gniewek P, Kolinski A. Coarse-grained modeling of mucus barrier properties. Biophysical journal. 2012;102: 195-200.
Doi:10.1016/j.bpj.2011.11.4010 - O’Brien TJ, Beard JB, Underwood LJ, Dennis RA, Santin AD, York L. The CA 125 gene: an extracellular superstructure dominated by repeat sequences. Tumor Biology. 2001;22: 348-66
Doi:10.1159/000050638 - Blalock TD, Spurr-Michaud SJ, Tisdale AS, Heimer SR, Gilmore MS, Ramesh V, Gipson IK. Functions of MUC16 in corneal epithelial cells. Investigative Ophthalmology & Visual Science. 2007;48: 4509-18.
Doi:10.1167/iovs.07-0430 - Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. 2010;29: 2893-2904.
Doi:10.1038/onc.2010.87 - Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutrition Journal. 2010;9:2-16.
Doi:10.1186/1475-2891-9-69 - Göcze P, Vahrson H. Ovarian carcinoma antigen (CA 125) and ovarian cancer (clinical follow-up and prognostic studies). Orvosihetilap. 1993;134: 915-18.
PMID:8479736 - Santillan A, Garg R, Zahurak ML, Gardner GJ, Giuntoli RL, Armstrong DK, Bristow RE. Risk of epithelial ovarian cancer recurrence in patients with rising serum CA-125 levels within the normal range. Journal of Clinical Oncology. 2005;23: 9338-43.
Doi:10.1200/JCO.2005.02.2582 - Mais DD, Leonard GR. Quick Compendium Companion for Clinical Pathology (2nd ed.). Chicago: American Society for Clinical Pathology. 2009; 352
- Baron JA. Screening for cancer with molecular markers: progress comes with potential problems. Nature Reviews Cancer. 2012;12: 368-71.
Doi:10.1038/nrc3260 - Nossov V, Amneus M, Su F, Lang J, Janco JMT, Reddy ST, Farias-Eisner R. The early detection of ovarian cancer: from traditional methods to proteomics. Can we really do better than serum CA-125? American journal of obstetrics and gynecology. 2008; 199: 215-23.
Doi:10.1016/j.ajog.2008.04.009 - Fritsche HA, Bast RC. CA 125 in ovarian cancer: advances and controversy. Clin Chem. 1998; 44:1379–80.
PMID:9665412 - Bai H, Sha G, Xiao M, Gao H, Cao D, Yang J, Chen J, Wang Y, Zhang Z, Shen K. The prognostic value of pretreatment CA-125 levels and CA-125 normalization in ovarian clear cell carcinoma: a two-academic-institute study. Oncotarget. 2016;7: 15566-76.
Doi:10.18632/oncotarget.7216 - Pradjatmo H, Pradjatmo H. Impact of preoperative serum levels of CA 125 on epithelial ovarian cancer survival. Asian Pac J Cancer Prev. 2016;17: 1881-86.
Doi:10.7314/apjcp.2016.17.4.1881 - Furrer D, Grégoire J, Turcotte S, Plante M, Bachvarov D, Trudel D, Têtu B, Douville P, Bairati I. Performance of preoperative plasma tumor markers HE4 and CA125 in predicting ovarian cancer mortality in women with epithelial ovarian cancer. PloS one. 2019;14: e0218621.
Doi:10.1371/journal.pone.0218621
Department of Gynaecological Oncology, Bangabandhu Sheikh Mujib Medical University, Shahbagh, Dhaka, Bangladesh.
shirin.bsmmu@gmail.com
 0000-0001-5621-1390
0000-0001-5621-1390
Submission
2020-11-08
Accepted
2021-06-30
Published
2021-08-01
Apply citation style format of Bangladesh Medical Research Council
Issue
Vol 47 No 2 (2021)
Section
Research Articles
Financial Support
None
Conflict of Interest
There is no conflict of Interest