Nasrin Hossain
Department of Gynecological Oncology, National Institute of Cancer Research and Hospital, Dhaka, Bangladesh.
Farhana Tarannum Khan
Department of Gynecological Oncology, National Institute of Cancer Research and Hospital, Dhaka, Bangladesh.
Mohammad Sharif Mahmud
Combined Military Hospital, Dhaka Cantonment, Dhaka, Bangladesh.
Keywords: Epithelial ovarian cancer, Disease free survival, FIGO stage, Non-epithelial ovarian malignancy
DOI: 10.3329/bmrcb.v46i2.49021
Abstract
Background: Ovarian cancer is the ninth most common cancer in women and it ranks fifth as the cause of cancer death in women. Epithelial ovarian cancer (EOC) represents the most lethal malignancy of the female genital tract. EOC is typically diagnosed in advanced FIGO (Federation of International Gynaecology and Obstetrics) stages due to lack of symptoms in the earlier stages. So, the aim of this study was to determine the disease free survival (DFS) of EOC after treatment and also to find out the prognostic factors such as age, FIGO stage, histopathological subtypes and grade for recurrence of disease.
Methods: A retrospective study was conducted in gynaecological oncology department of National Institute of Cancer Research and Hospital (NICRH), Dhaka. Study period was 02 years from January 2017 to December 2018.Histopathologically confirmed epithelial ovarian malignancy after completion of treatment was included in this study. Non-epithelial ovarian malignancy was excluded from this study.
Results: Mean age was 53 yrs (range 25-80 yrs).Final surgical staging (FIGO stage) of disease were as follows stage I; 24 (25.8%), stage II; 18 (19.4%), stage III; 45 (48.4%) and stage IV; 6 (6.5%). Mean follow-up time was 34 months (range 24-60 months). DFS according to FIGO stages were 35 months, 27 months, 25 months and 9 months respectively. DFS based on the complete and incomplete surgical staging were 27 months and 22 months respectively. At univariate analysis, factors significantly associated with decrease DFS and increase recurrence which included age (HR- 1.39 95% CI), stage (HR- 1.22, 95% CI), histopathological type (HR-1.28, 95% CI) and grade of tumor (HR-1.65, 95% CI). At multivariate analysis, FIGO stage (HR-3.9, 95% CI), histopathological type (HR-1.86, 95% CI) and grade (HR-1.11, 95% CI) having an association with decease DFS.
Conclusion: Surgical stage was one of the strongest independent prognostic factors of DFS for EOC. Increasing age, histopathology and grade are also independent predictors of decrease DFS in patient with EOC treated with current standard therapy.
Keywords: Epithelial ovarian cancer, Disease free survival, FIGO stage, Non-epithelial ovarian malignancy
Introduction
Ovarian cancer is the ninth most common cancer in women and it ranks fifth as the cause of cancer death in women.1,2 It accounts for 4% of all malignancies affecting females.3 They also constitute 40% of all gynaecological malignancies.2 Women are at risk of developing ovarian cancer during their life time is 1.5% of them.1
Epithelial ovarian cancer (EOC) represents the most lethal malignancy of the female genital tract. EOC is typically diagnosed in advanced stage. FIGO (Federation of International Gynaecology and Obstetrics) stages of ovarian cancer is surgico-pathological staging. Most of the patients with ovarian cancer are stages III- IV due to lack of symptoms in the earlier stages. Ovarian malignancy usually disseminated to the peritoneal cavity and spread outside the abdominal cavity is a rare and late event.5
According to Globocon ovarian cancer rank is 12th, 3063 new cases detected.³ Prevalence of ovarian cancer, 5 year prevalence all ages are 7.99/100000.3 According to NICRH statistics from last 5 years (2014-2018), total number of ovarian malignancy was 6114, which represents 22% of gynaecological malignancy.4
There are different histopathological types of ovarian tumors according to cell of origin. EOC are the most common type as they account for 80-90% of ovarian tumors.6 Grade of tumors varies border line or low malignant potential to tumors of high grade or undifferentiated tumours.6
Diagnosis of ovarian tumor is depends mainly on the clinical presentation of the patients, laboratory investigations and imaging modalities.6
One Italian study reported that shorter disease free interval is significantly associated with age, stage and suboptimal cytoreduction.4 One American study showed that disease free survival is independent prognostic factor of increasing age, performance status, histology and residual disease. Another USA study found that multivariate regression analysis revealed that stage IV, histology, malignant pleural effusion, intra-parenchymal liver metastasis and residual tumor size were significant prognostic variables for disease free survival and overall survival.7
Basic surgical procedures used in the management of malignant ovarian tumors include: Staging laparotomy, primary completion surgery, interval debulking surgery (IDS), secondary cytoreduction, exploration with biopsy. Now for ovarian cancer standard treatment is the primary surgical treatment, which is usually followed by chemotherapy.1
Prognosis of ovarian cancer depends mainly on the stage of the disease. Five years survival rate for carefully staged IA disease is about 90% while it is 70-80% for stage IC.6 The 5yrs survival for stage II disease is about 70%, 45-50% for stage IIIA, 40% for stage IIIB, 30-35% for stage IIIC and 15-20 % for stage IV disease.7
A worse prognosis is associated with some histological types like // clear cell carcinoma, high grade tumors, increased aneuploidy and with some oncogene expression: Ras, c-erb-b-2 and myc. The present study was carried out to find out the disease free survival (DFS) and any association with prognostic factors such as stage, grade, histopathology and incomplete surgical staging.
Materials and Methods
It was a retrospective cohort study conducted in gynaecological oncology department of National Institute of Cancer Research and Hospital (NICRH) during the period of January 2017 to December 2018. Sampling technique was purposive. An inclusion criterion in the study was primary epithelial ovarian cancer after treatment (surgery, chemotherapy or both). Non epithelial ovarian cancer was excluded from this study. Formal permission was taken from the ethical committee of the NICRH, Dhaka. Informed consent was taken from the patients. Complete history, physical examination and relevant investigations were done. Data were collected in a pre-designed data collection sheet. Clinical and pathological data were collected from medical records, pathology reports and cancer registry reports. Every patient was evaluated by history taking and complete physical examination. If there was any suspicious of recurrence on clinical evaluation, than USG/CT scan of whole abdomen and pelvis and chest-X ray was advised. All recurrence was confirmed by tissue diagnosis. Data were analyzed using SPSS software. A p value < 0.05 was regarded as statistically significant.
Results
A total of one hundred and fifty patients with epithelial ovarian cancer (biopsy confirmed) were included in this study. The mean age was 53 yrs with a range of 25-80 yrs (table I).
Final stages of disease were as follows: stage I; 24(25.8%), stage II; 18 (19.4%), Stage III; 45 (48.4%) and stage IV; 6 (6.5%). The distributions according to histopathological subtypes were as follows: serous 124 (82.7%), mucinous 16 (10.7%), endometroid 8 (5.3%) and clear cell carcinoma 2 (1.3%). The majority of patients 72 (48.0%) had grade I, while 30.7% had grade II tumors and 14.7% had grade III tumors (table I). Surgical procedures included: primary surgery (complete surgical staging) was 62 patients (41.3%), NACT followed IDS were 31 (20.8%) and incomplete surgical procedure were 57 (38.0%) (table II).
| Age | Number of patients | Percentage (%) |
|---|---|---|
| Mean age 53 yrs | Range 25-80 yrs | |
| Surgical staging | ||
| Stage I | 24 | 25.80 |
| Stage II | 18 | 19.35 |
| Stage III | 45 | 48.38 |
| Stage IV | 6 | 6.45 |
| Histopathological type | ||
| Serious adenocarcinoma | 124 | 82.7 |
| Mucinous adenocarcinoma | 16 | 10.7 |
| Endometroid carcinoma | 8 | 5.3 |
| Clear cell carcinoma | 2 | 1.3 |
| Grading (differentiation) | ||
| Well differentiated | 72 | 48.0 |
| Moderate differentiated | 46 | 30.7 |
| Poorly differentiated | 22 | 14.7 |
| Surgical procedure | Number of patients | Percentage (%) |
|---|---|---|
| Primary Surgery (Surgical Staging) | 62 | 41.33% |
| NACT Followed by IDS | 31 | 20.8% |
| Incomplete surgical staging | 57 | 38% |
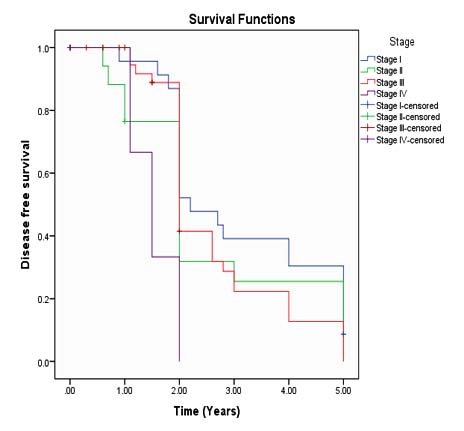
Mean follow-up time was 34 months (range 24-60 months). Mean disease free survival (DFS) according to FIGO stages were 35 months, 27 months, 25 months and 9 months respectively (Figure 1).
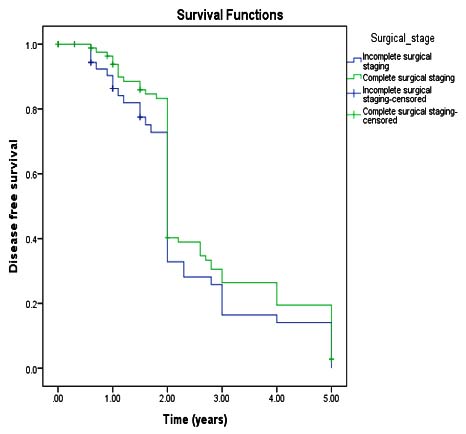
Mean DFS in complete and incomplete surgical staging were 27 months and 22 months respectively but statistically not significant (p value-0.173).At univariate analysis, factors significantly associated with decreased DFS and increased recurrence which included: age (Hazard Ratio-1.39 95% CI), advanced stages (HR-1.22 95% CI), histopathological type (HR-1.28 95% CI), and grade of tumor(HR-1.65 95% CL) (Figure 2).
| Characteristics | Total Number | Recurrence | Hazard ratio | p value |
|---|---|---|---|---|
| Age in years | No (%) | No (%) | ||
| ≤50 | 38(25.3%) | 10 (26.3%) | 1.391 |
0.383 |
| >50 | 112(74.7) | 44(39.3%) | ||
| FIGO Stages | ||||
| Stage I | 24(25.8%) | 4(16.7%) | 1.22 |
0.529 |
| Stage II | 18(19.4%) | 10(55.6%) | ||
| Stage III | 45(48.4%) | 17(37.8%) | ||
| Stage IV | 6(6.5%) | 2(33.3%) | ||
| Histopathological type | ||||
| Serious adenocarcinoma | 124(82.7%) | 52(41.91%) | 1.280 |
0.447 |
| Mucinous adenocarcinoma | 16(10.7%) | 7(43.75%) | ||
| Endometroid carcinoma | 8(5.3%) | 3(37.50%) | ||
| Clear cell carcinoma | 2(1.3%) | 1(50.00%) | ||
| Grading | ||||
| Well differentiated | 72(48%) | 21(36.8%) | 1.653 |
0.046 |
| Moderate differentiated | 46(30.7%) | 23(40.4%) | ||
| Poorly differentiated | 22(14.7%) | 13(40.4%) |
Increasing of age was associated with increasing recurrence, based on FIGO stages that advanced stage associated with increased recurrence and based on tumor histopathology, endometriod (recurrence rate 37.5%) had improved clinical outcome compared with serous tumor (recurrence rate 41.9%). Mucinous and Clear cell had increased recurrence which were 43.7% and 50.0% respectively (table III). At, multivariate analysis stage of disease (HR-3.9 95% CI), histopathological type (HR-1.86 95%CI), histopathological grade (HR-1.1 95%CI) having an independent association with decrease DFS (table IV).
| Disease free | Recurrence | |||
|---|---|---|---|---|
| HR | p value | HR | p value | |
| Stage | 3.979 | 0.008 | 1.221 | 0.529 |
| Histopathological type | 1.862 | 0.369 | 0.865 | 0.742 |
| Grade | 1.145 | 0.785 | 0.961 | 0.905 |
| Incomplete surgical staging | .000 | 0.985 | 0.620 | 0.650 |
Discussion
Diagnosis and management of ovarian cancer is one of the most important challenges in oncology because it is the leading cause of death among all gynaecological malignencies.1 Most women are usually diagnosed with advanced disease with subsequent difficulty in treatment with high rate of recurrence and bad prognosis.5 Primary cytoreductive surgery followed by platinum based multiagent chemotherapy is utilized by gynaecologic oncologists for managing the vast majority of patients with advanced epithelial ovarian cancer.6
In this study, total 150 patients were included. 93 patients had complete surgical staging information, among them most common surgical stage was stage III (48.38%) than stage I, stage II and stage IV were 25.80%, 19.35% and 6.45% respectively. 57 patients had incomplete surgical staging information. All patients were completed chemotherapy after surgery. Many studies reported similar findings.10,11 Stage IIIC was most common presentation than other stages. Other studies have stated that complete surgical staging without residual disease have a good prognostic value than incomplete surgical staging/ complete surgical staging with residual disease.4,7
In current study, histopathologically serous was most common (82.7%) than mucinous (10.7%), endometriod (5.3%) and clear cell (1.3%) types. In this study, we used three tires system of grading, most common grade I (48%), next grade II (30.7%) and grade III (14.7%).These findings are consistent withother studies.5, 7, 13
Findings this study suggested that older women are at increased risk of recurrence (HR-1.391). One study showed differences in tumour biology, immune response and co-morbidities may explain the poorer prognosis in elder patients.7, 14
In the current study, FIGO stage was an independent significant prognostic factor for disease free survival (HR-3.939 95% CI) (p< 0.008). Mean follow-up time was 34 months and range was 24-60 months. Disease free survival (DFS) according to FIGO stages; stage I, Stage II, stage III and stage IV were 35 months, 27 months, 25 months and 9 months respectively Several other studies reported that FIGO stage was an independent significant predictor for DFS. In this study, multivariate analysis showed histological subtypes (HR-1.862) and histopathological grade (HR- 1.145) were independent prognostic factor for DFS but statistically not significant (p-value< .396 & .785 respectively).4, 11, 15
In this study, reported that univariate analysis, histopathological types(HR-1.28095% CI) and grade (HR-1.653 95% CI) was independent significant for recurrence and grade was statistically significant (p<.040). This study showed that mucinous (43.8%) and clear cell(50.0%) subtypes had more recurrence when compared with serous (41.9%) histology. Studies reported that clear cell and mucinous histopathology have been generally accepted as an unfavourable histopathology.7, 13,14 In multivariate analysis did not confirm that histopathology and grade were significant for recurrence (HR-.865 95% CI) and (HR-.961 95% CI) respectively.
Several other studies reported that tumor grade was a significant prognostic variable in the early stage EOC; it does not appear to be a predictor of poor outcome advanced stage disease.14,11 It was reported that grade was not an independent predictor of outcome. This finding is dissimilar to current study finding.13
In the current study, mean DFS in case of incomplete surgical staging was 19 months compared with complete surgical staging was 24 months but not statistically significant (p valve <.173). It is contrast to one study that reported incomplete surgical staging was a significant prognostic factor for DFS.15
The study had several limitations that must be acknowledged. First the retrospective nature of the study has inherent bias. Second small number of sample size, in addition the median follow-up was only 33 months which is a short period to determine the disease free survival.
Conclusion
Surgical stage represents one of the strongest independent prognostic biomarker of DFS for epithelial ovarian tumor. Increasing age, histopathology and tumor grade are all independent predictors of decreased DFS in patients with EOC treated with current standard therapy.
References
- Raja FA, Chopra N& Ledermann JA. Optimal first-line treatment in ovarian cancer. Journal of Oncology.2012;23;118-127.
Doi:10.1093/annonc/mds315 - Peiretti M, Zanagnolo V, Goivanni D et al. Role of maximal primary cytoreductive surgery in patients with advanced epithelial ovarian and tubal cancer: Surgical andoncological outcomes. Single institution experience. Journal of Gynecologic oncology. 2010;119:259-264.
Doi:10.1016/j.ygyno.2010.07.032 - Bangladesh source: Globocan 2018. International agency for research on cancer & WHO
- Cancer registry 2018, National Institute of Cancer Research and Hospital (NICRH), Dhaka, Bangladesh.
- William E, Winter III, G. Larry Maxwell et al. Prognostic Factors for Stage III Epithelial Ovarian Cancer: A Gynaecologic Oncology Group Study. Journal of Clinical Oncology.2007;25: 3621-3627.
Doi:10.1200/JCO.2006.10.2517 - Jonathan S. Berek, Neville F. Hacker. Berek & Hacker’s gynaecological oncology. 6th edition, Philadelphai; Wolters Kluwer.2015.pp 54-678.
- Wiliam E, Winter III, G. Larry Maxwell et al. Tumor Residual after Surgical cytoreduction in Prediction of Clinical outcome in stage IV Epithelial Ovarian Cancer: A Gynecologic Oncology Group Study. Journal of Clinical Oncology. 2008;26:83-89.
Doi:10.1200/jco.2007.13.1953 - David I, Esther N, Robert A, et al. Are patients willing to travel for better ovarian cancer care? Journal of gynaecologicOncology.2018;148:42-48.
Doi:10.1016/j.ygyno.2017.10.018 - Frank L, David L, Charles R,et al. Feasibility and Outcome of Complete Secondary Tumor Resection for Patients with Advanced Ovarian Cancer.Journal of Surgical Oncology. 1990; 45:14-19.
- El-Sayed A, Ibrahim F, Alaa Y, et al. Cytoreductive Surgery for Advanced Epithelial Tumors of the ovary: Technical Considerations and outcome. Journal of the Egyptian Nat. Cancer Inst. 2005; 17:158-164.
- Robert E, Bristow, Rafael S, et al. Survival Effect of maximal Cytoreductive Surgery for Advanced Ovarian Cancinoma During the Platinum Era: A Meta-Analysis. Journal of clinical Oncology. 2002;20:1248-1259.
Doi:10.1200/JCO.2002.20.5.1248 - Scott M, Nick M, Spirtos, et al.Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: a prospective study. Journal of Gynecologic Oncology. 2003;3:390-396.
Doi:10.1016/s0090-8258(03)00278-6 - Anna F,Gabriella F, Giuseppe V, et al. Phase III randomized clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumor load (SCORPION trial): Final analysis of peri-operative outcome. European Journal of cancer. 2016;59:22-33.
Doi:10.1016/j.ejca.2016.01.017 - Junor E, Hole D, Mcnulty L, et al. Specialist Gynaecologists and survival outcome in ovarian cancer: a Scottist national study of 1866 patients..BJOG: international Journal of Obstetrics & Gyneecology.2005;106: 147-163.
Doi:10.1111/J.1471-0528.1999.tb08137 - Kevin H, Kayla M, Kristen S, et al. Prognostic value of miliary versus non-miliary sub-staging in advanced ovarian cancer. Journal of Gynaecology oncology. 2017;146:52-57.
Doi:10.1016/j.ygyno.2017.05.005 - AwwL, Mesher D, Gentry- Maharaj A, et al. Time to diagnosis of Type I or II invasive epithelial ovarian cancers: a multicentre observational study using patient questionnaire and primary care records. An international Journal of Obstetrics and gynaecology (BJOG). 2016;123: 1012-1020.
Doi:10.1111/1471-0528.13447
Department of Gynecological Oncology, National Institute of Cancer Research and Hospital, Dhaka, Bangladesh.
nasrinhossin23@gmail.com
 0000-0002-0467-434x
0000-0002-0467-434x
Submission
2019-03-20
Accepted
2019-12-01
Published
2020-08-01
Apply citation style format of Bangladesh Medical Research Council
Issue
Vol 46 No 2 (2020)
Section
Research Articles
Financial Support
None
Conflict of Interest
There was no conflict of interest.